Summary
- Metrological verification of air particle counters: constructing a test bench to measure counting efficiency according to ISO 21501-4.
- On the importance of monitoring the parameters of the treated water supply used in the manufacture of water for pharmaceutical use on production premises.
- New technologies for single-use biopharmaceutical process flow measurement
- The Calibration of Embedded Sensors
- Cleaning and disinfection - A one or a two steps process or scientifically justified ?
- Updated regulations on FDA acceptance of medical device clinical data in effect soon.
The draft European Good Manufacturing Practice (EU GMP) Annex 1 is the focus of a great deal of discussion. In many ways this is good as it builds interest, engagement, and inclusion. On the downside, there is potential to spread misinformation. It would be prudent for pharmaceutical manufacturers to practice due diligence before changing any procedures. The goal of regulatory guidance is to provide standards for basic requirements, not to define procedures. This allows manufacturers to develop and implement procedures that are tallored to their own facilities and processes.

The procedures must be designed according to each manufacturer’s unique processes, rather than simply adhering to the minimum regulatory standard, which may or may not be appropriate and can result in misaligned procedures. An effective procedure can only be designed based on a scientific approach that is aligned with the regulatory requirements.
The objective of this article is to clarify when a one or a two-step cleaning and disinfection procedure (for clean rooms and non-contact product surfaces) should be implemented. In other words, is a separate and distinct cleaning step always needed prior to disinfection?
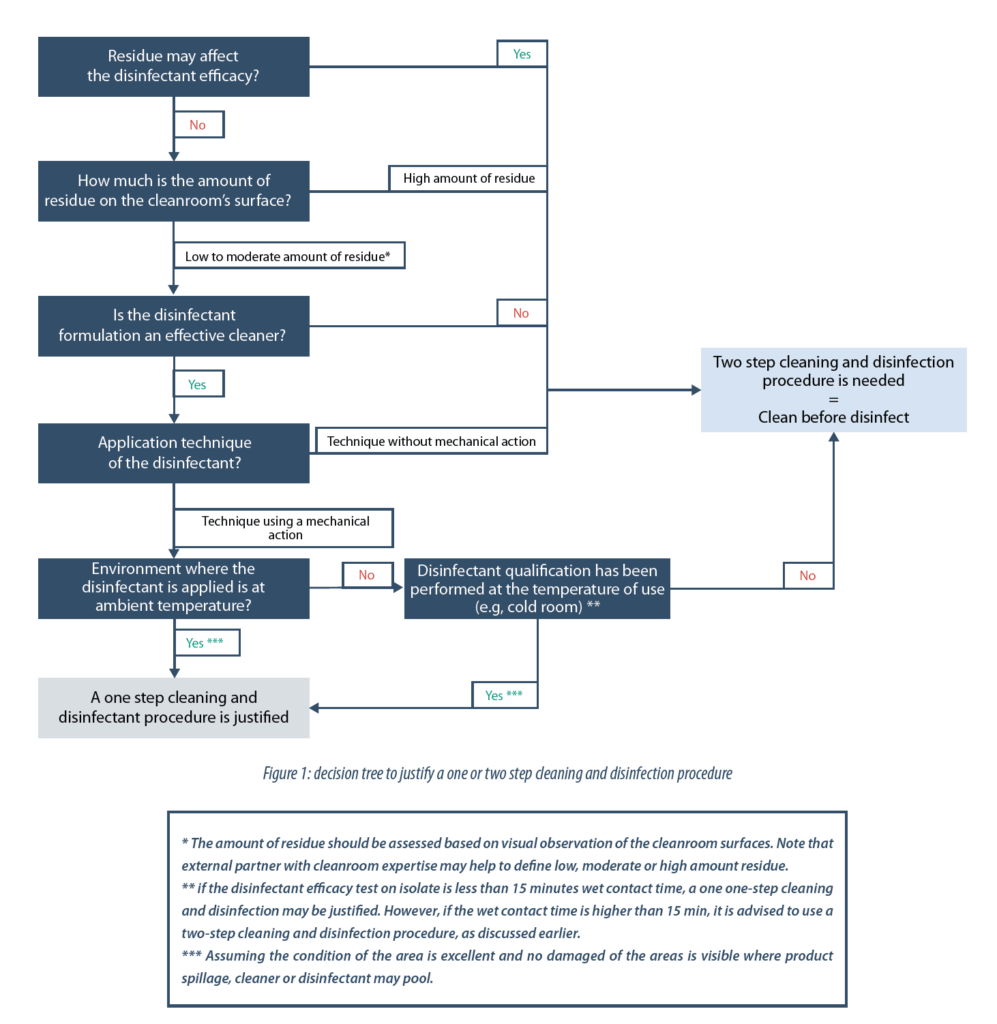
1. A one or a two-step cleaning and disinfection procedure
“Clean before disinfect” is the golden rule that everyone knows and a legacy from the healthcare and food industry (1,2) which often relies on bleach, which cannot handle large amounts of soil load and which has limited stability. Further, their processes can generate high amounts of organic and inorganic residue(1) which can rapidly deplete products that are not designed to handle such soil. In these cases, the cleaning step aims to remove visible soils from the surfaces, prior to disinfection, to avoid interference with the disinfectant’s effectiveness(1). Therefore, cleaning prior to disinfection is necessary for a manufacturing process that generates a high visible amount of residue.
High levels of residue may be generated in production of API’s (active pharmaceutical ingredients), tablets or liquid-dose manufacturing cleanrooms where the containment systems may be inadequately designed. In this instance, it is not possible to avoid the accumulation of residues on surfaces.
However, cleanrooms designed with proper containment systems, such as closed manufacturing or hood systems, help to reduce or prevent the build-up of visible residues. Additionally, there are numerous manufacturing processes that generate very low levels of residue. In such cases, is a one-step cleaning and disinfection procedure possible?
The amount of residue generated should not be the only parameter that dictates a cleaning and disinfection program. The source and nature of the residue should be considered as well. In particular, organic material, for example from a media spill, may present more of a challenge to a formulated disinfectant than light soil from personnel motion e.g. walking, in particular in highly restricted areas. It is evident that different parameters must be considered before implementing a cleaning and disinfection procedure:
1.1. Interaction of the residues
If a residue is known to have no adverse interaction with the disinfectant efficacy, then a cleaning step is not necessary. However, if an interaction is known, a separate cleaning step must be performed prior to disinfecting.
1.2 Amount of the residues
As discussed earlier, a cleaning step is key to removing high amount of visible material prior to disinfection. However, in some cases and depending on the disinfectant composition, a low level of residue may not need to have a separate cleaning step prior to disinfection (refer to point 3 below). Note that one can argue how to define low level (“clean” cleanroom) or high-level (“dirty” cleanroom) amounts of residue. This should be decided based on field observations.
1.3 Disinfectant composition
The composition of the disinfectant is a key parameter to consider before developing a cleaning and disinfection procedure. Certain disinfectants formulated with surfactants are known to be capable of cleaning and disinfecting in one step. In addition, the mechanical action (e.g. wiping, mopping) using such formulated disinfectants helps to keep surfaces clean. At the same time, the disinfection process is performed by maintaining the wet contact time confirmed during in vitro testing.
Note that the in vitro surface coupon testing separates disinfection from cleaning, focusing on the chemical kill (disinfection) of the product. According to the U.S. EPA, “an antimicrobial agent identified as a ‘one-step’ cleaner-disinfectant, cleaner-sanitizer, or one intended to be effective in the presence of organic soil must be tested for efficacy by the appropriate method(s) which have been modified to include a representative organic soil such as 5% blood serum”(3). However, the EPA also suggested to simulate and to confirm the disinfectant capability to clean in real conditions where the disinfectant is used(3).
1.4 Disinfectant application technique
Heavily soiled or “dirty” cleanroom surfaces should be cleaned when a disinfectant is applied without mechanical actions such as spraying or vaporizing. This is applicable even if the disinfectant contains surfactant.
As discussed earlier, mechanical action using a formulated disinfectant helps to clean while disinfecting. However, a disinfectant without surfactants, e.g. 5% bleach, 3% H2O2, would fall into a grey area, where it may not be as effective for cleaning, even with mechanical action. Other factors should also be analyzed such as the choice of the material of construction of the mop, or wipe that may play a role in removing residue to acceptable level. The volume of disinfectant solution being used to mop, or wipe may have an impact on disinfectant residue build-up. Therefore, whether the surface is being cleaned and disinfected with a saturated, high liquid retaining mop/wipe or a flood of disinfectant in an attempt to really wet the area plays a role in residue development.
1.5 Environment of use
Disinfectant efficacy studies are typically performed at an ambient temperature. However, in cold room processes, the necessary wet-contact times might be as much as 3X higher, depending on temperature and the target microorganisms. In such cases, cleaning prior to disinfection may help to reduce the bioburden level and may justify shortening the disinfection contact time. In cold-room area, the traffic is generally limited and therefore low frequency of cleaning and disinfection may be implemented, if scientifically justified.
Finally, other factors such as the condition of the area is important to assess, for example if a floor is damaged and there are areas where product spillage or disinfectant may pool, higher residue is to be expected.
The manufacturer should analyze the different parameters examined earlier. The outcome of the evaluation should help to draw a scientific justification for deciding whether a one or two step cleaning and disinfection procedure is required. Below are two approaches manufacturers may use to determine if a one or two-step cleaning and disinfection process is needed:
- In some cases, for new facility or when not enough historical data or observations are available, a simple decision tree may help in determining whether a one or two step cleaning and disinfection procedure is justified:
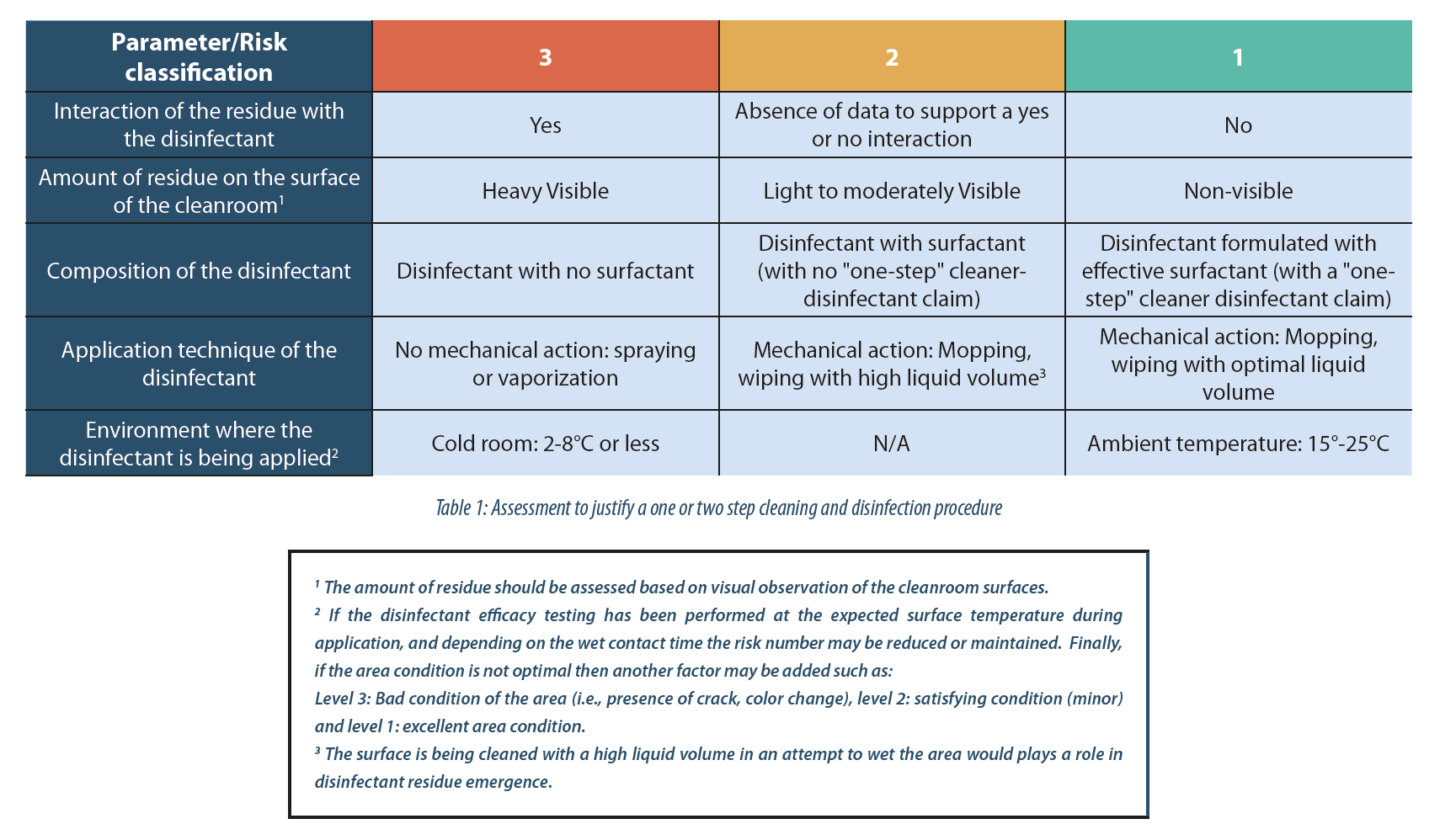
- When enough historical data or observations are available; a numeric assessment may be possible. Below is an example of a simple assessment approach which may help justify a one or two step cleaning and disinfection procedure:
For the sake of simplification, the risk classification used is 1 (low risk), 2 (medium risk) and 3 (high risk).
In this case, the Risk Priority Number (RPN) is equal to the sum of the value of each parameter. Therefore, a lower RPN equal to 5, a medium RPN equal to 8 and a higher RPN equal to 15. Note that based on historical data and observations, the manufacturer should be able to set up the RPN threshold value themselves. In this example a threshold value is 7:
- RPN value higher than 7: a separate cleaning step may be needed prior to disinfection. This means, even if the disinfectant contains surfactant in their formulation, a two-step cleaning and disinfection procedure is desirable.
- RPN value between 5 and 8, or equal to 8: a separate cleaning step is not required prior to disinfection. However, if the environment where the disinfectant is applied includes damaged areas where product spillage, cleaner or disinfectant may pool, or includes a cold room, and depending on the microorganism, a cleaning step may be beneficial.
- RPN value lower than or equal to 5: a separate cleaning step is not essential prior to disinfection. Therefore, a one-step cleaning and disinfection procedure is acceptable.
The complete set of factors that affect cleaning and disinfection effectiveness should be identified and considered. An analysis of the factors with “in the field” observation is necessary to understand the different factors that impact the effectiveness of the cleaning and disinfection program. Finally, a thorough analysis of the manufacturer’s surface cleanroom “cleanness”, the technique to apply the disinfectant, the disinfectant composition, and the environment where the disinfectant is used, is crucial to support scientific justification of a one or two-step cleaning and disinfection procedure.
Share article

Walid EL AZAB – STERIS
walid_elazab@steris.com
References
(1) William A. Rutala, David J. Weber and the Healthcare Infection Control Practices Advisory Committee, Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
(2) HTA, Food Standards Agency, E. coli guidance update, access on September 24,2018
(3) Environmental Protection Agency, Pesticides: science and policy: efficacy data requirements supplemental recommendations, access on October 18,2018: https://archive.epa.gov/pesticides/oppad001/web/html/dis-02.html