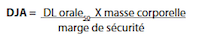Summary
- Cluster consolidation strategies for solid oral forms in multi-product establishments
- Biocidal regulations applied to the disinfectants used in the pharmaceutical industry: all you need to know…
- Cryogenics is the study and production of low temperatures
- Cleaning Validation for biotechnological substances : What acceptance criteria ?
- “Health-based approach” implementation for setting limits in cleaning validation for Vaccines/Biotech
To this day, oral formulations are the largest category of pharmaceutical and nutritional dosage forms. Hundreds of manufacturers around the world produce thousands of products in tablet, capsules, and powder forms which are often referred as Oral Solid Dosage (OSD) products.

In the United States of America (USA), the cGMP regulation for pharmaceutical products states that “equipment and utensils shall be cleaned, maintained, and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality or purity of the drug product”(1). Similarly, in June 2007, the US Department of Health and Human Services, the US Food and Drug Administration (FDA) has issued regulations for dietary supplements.
This regulation states that manufacturers “must maintain, clean, and sanitize, as necessary, all equipment, utensils, and other contact surfaces used to manufacture, package, label, or hold components or dietary supplements” (2) . This being said, all OSD manufacturers supplying products to the USA must have a cleaning program in place to comply with these regulations.
FOS products contain one or more active ingredients that can be classified as pharmaceuticals (eg, paracetamol, ibuprofen, etc.) or food products (eg, vitamins, minerals, plants, etc.). Depending on the product, it may contain several excipients (inert ingredients) used as fillers, disintegrants, bonding agents, lubricants, dyes and / or coatings. The wide variety of active substances and excipients as well as product variations make it difficult for companies to define a single cleaning strategy for FSO manufacturing operations, making it more difficult to comply with cGMP regulations.
Grouping strategies, also known as matrix or bracketing is a method by which products or equipment are considered to be similar for the purposes of cleaning. If the products are of similar product type, manufactured in the same equipment train, and cleaned by the same cleaning methods, then grouping strategies can be used to simplify the cleaning procedure that is intended for multiple products. Products are grouped based on product type (tablet, gel capsule, soft gel, etc.), solubility and toxicity of the active ingredient(s), and overall difficulty of cleaning based upon historical or laboratory data. Process equipment is grouped based on its design and size. In either case, a worst-case product or piece of equipment is selected to perform the cleaning challenge. This article addresses the selection of the worst-case product grouping for OSD forms, and reviews data relating to solubility, toxicity and cleaning difficulty. The article also includes laboratory experiments on final formulations to assess cleanability using vitamins and minerals as an example.
Solubility of OSD Products
Solubility data for the active component in water is often easy to generate or obtain (see examples in Table XNUMX). When a cleaning agent other than water is used the same type of data should be collected. Generally, organic solvents are not common cleaning agents used by OSD manufacturers due to the difficulty of eliminating solvent residues plus environmental and personnel safety concerns. Most OSD manufacturers perform cleaning by manual methods (e.g. brush, wipes, foam or spray wands, etc.) with a formulated aqueous cleaning agent.
It is often assumed that the active substance with the lowest solubility is the most difficult to clean and is therefore the “worst case”, but several factors may complicate this assumption. First, the active is just one of many components in an OSD product formulation. The other components, known as excipients, fillers or inactive ingredients (see examples in Table XNUMX), may influence the solubility of the active. Second, often the excipents are harder to clean than the active. Third, parameters such as the pH and temperature of the cleaning solution can impact the solubility of the active. In addition, where a formulated detergent is used, the solubility of the active may be improved due to the multiple components in the cleaning formulation (e.g. acid, base, chelant, dispersant or surfactant). Anyhow, the solubility of the active can be useful data from a risk assessment perspective to determine the worst-case soil.
Toxicity of OSD Products
In the pharmaceutical industry, acceptance limits for residual actives are often calculated using the minimum daily dose of the active in the product just manufactured, and the maximum daily dose of the next product to be manufactured. This limit calculation is based on the smallest therapeutic dose, and it has been accepted industry-wide by most drug manufacturers in establishing scientifically justifiable limits for cleaning validation. The term “minimum daily dose” refers to the minimum prescribed dose of active that could cause the desired pharmacological effects in a patient. A safety factor of 0,001 has been recommended for oral dosage forms (5).
Since dietary supplements are not drug products, the application of a pharmaceutical calculation may not be relevant because pharmacological information is not available for most dietary actives used in these products. Another measure, such as the recommended dietary allowance, may be listed on the product label or in other references but these recommended values may vary greatly. Dietary supplements can be taken for multiple purposes, and dosage can depend on individual needs, so nutritionist recommendations may differ. Therefore recommended dietary allowances are not an appropriate substitute for the therapeutic dose data as in the case of drug products. Other residual limit calculation options may be considered instead.
An alternate method of calculating acceptable limits is based on the use of animal toxicity data in place of the minimum daily dose. This method is particularly useful for establishing limits for substances that are not intended for therapeutic use. The toxicity information should include the route of administration, test species, and the dosage used. For example, oral DL50 (lethal dose for 50% of the population) in rats or mice is commonly available for comparison of oral dose products. Toxicity data like LD50 can be used to determine the acceptable daily intake (ADI) of a potential contaminant. The ADI takes into consideration the body weight (such as 25 kg for a child or 70 kg for an adult) to arrive at a “safe dose”. Such a calculation for a dietary supplement using the oral LD50 would then be:
In this equation, the appropriate safety margin is a combination of a safety factor (given in the reference as >1000,1 for prolonged or lifetime exposure to the drug) and a modifying factor (usually between 1 and 10, based upon the judgment of the toxicologist making the determination)(6-8). ADI could be used to calculate the maximum allowable carry-over (MACO) limit in the next product batch as :
From here, the rest of the residual limit calculation would follow a similar strategy as discussed in multiple references(5,9). The MACO value, together with the total shared surface area and/or final rinse volume, can be used to determine the residue limits for each swab or rinse sample, respectively. Oral toxicity data is generally available for FDA approved dietary actives (Table XNUMX). For actives or products with limited or no toxicity data available then an ADI can be based on the threshold toxicological concern (TTC) principle or using an acceptable daily exposure (ADE) or permitted daily exposure (PDE) (10-12). For product grouping, the acceptance limit for cleaning should be based on the lowest MACO value determined for the group of products(9). The toxicity data used to determine the acceptance limit should be carefully weighed with the most difficult-to-clean product(s)(13).
Cleaning Challenges of OSD
Some of the challenges encountered in cleaning OSD include process equipment design. Blenders, bins, granulators, driers and so on may not be designed to handle liquids and therefore clean-in-place (CIP) technology may not be an option without substantial modifications to the equipment. For most cases the process equipment must be dismantled and cleaned manually in sections. Manual cleaning procedures must take into consideration operator safety and what type of personal protective equipment (PPE) to wear to minimize exposure to process and cleaning agent residues. Also, substrate compatibility becomes a concern when cleaning parts made of different grades of hardened steel, which are susceptible to corrosion (e.g. tableting tools). As mentioned earlier, OSD are composed of various ingredients some of them being particularly challenging to remove from process equipment. Carbomers, a high molecular weight polymer, for example are very popular ingredients used in the manufacturing of OSD because of their versatility and generally recognized as safe (GRAS) status(36,37).OSD products may also contain polymeric coatings formulated for specific functions. These types of polymeric substances tend to dry onto the sides of processing vessels and to harden (like fingernail polish) which makes the removal of the polymer extremely difficult.
Determining the most difficult-to-clean product is based on solubility of the active, historical information from the operators on cleaning/removing the product from surfaces, and/or laboratory studies. The use of laboratory studies can help define critical cleaning parameters such as selection of cleaning temperature, time, cleaning agent and concentration. Laboratory study data can also help define standard cleaning procedures, which facilitates the grouping of products for cleaning. Laboratory- based cleanability studies can also help determine the hardest-to-clean residue for a given set of cleaning parameters.
Materials and methods
In preparation of laboratory test samples, tablets were crushed and caplets were opened and applied as a loose or compressed powder to 304 stainless steel coupons with a 2B surface finish. Liquid from inside soft gel caplets was applied directly onto the coupons. The different coupon applications simulate different residue conditions found in manufacturing.
The coated coupons were cleaned for a maximum of 60 min. or until they have reached the following cleaning results: Visually Clean (VP), without water film breakage and gravimetric mass less than or equal to 0,1mg of residue per coupon (approximately 0,5 – 1,0mcg / cm2). Figure 1 provides a visual illustration of a soiled coupon, a visual failure and a water break-free test failure.
Figure 1: A dirty coupon on the left; failure of the visual test in the center; a failure in the water film rupture test on the right.
Visually clean – The cleanliness test is confirmed using large stainless steel coupons (usually 3 ″ x 6 ″). Once the cleaning test has been carried out, the coupon is rinsed with tap water for 10 seconds and then its cleanliness is observed. A coupon is considered visually clean if no residue of the sample or detergent is visible on either side of the coupon. Without water film rupture – A visually clean coupon is rinsed with deionized water and subjected to the water film rupture test. The surface of the coupon is coated with deionized water for 10 seconds in the vertical position and it is examined while the film of water is flowing. If the surface is clean, the water forms a thin continuous film which uniformly coats it. This film will persist for 30 to 60 seconds. Pre / post-cleaning weight – A visually clean coupon that has been subjected to the water film rupture test is air dried at room temperature and then weighed on an analytical balance to determine the post-cleaning weight. The post-cleaning weight is compared to the pre-coating weight obtained for the clean and dry coupon before coating with a sample. A large coupon is considered 100% clean by weight if the pre- and post-cleaning weights are equal to 0,1 mg.
The laboratory cleaning studies were performed using the agitated immersion method to compare various aqueous cleaning agents. Although manual cleaning is commonly used in the dietary supplement industry, agitated immersion was selected to eliminate the operator variability of typical manual cleaning methods. In addition, the agitated immersion method can also help evaluate the actual performance of cleaning chemistries because minimum mechanical force is employed.
The cleaning agents evaluated were: de-ionized (DI) water, neutral pH detergent, acid detergent, and alkaline detergent. The temperatures used in the study included ambient temperature (20-25°C), 45°C and 60°C. If a coupon was successfully cleaned at a lower temperature, then the higher temperature was not evaluated (for manual cleaning, typically temperatures of 45°C or less are recommended). The concentration of the selected formulated detergent was set to 1% v/v. Low effective detergent concentrations are desirable since they may impact operator safety, reduce the volume of neutralizer needed (if applicable), minimize the volume of rinse water needed, and optimize the effective use of the formulated detergent. Finally, contact times between the soils and the cleaning solution were increased (up to 60 minutes maximum) until desired results were achieved.
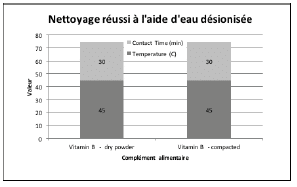
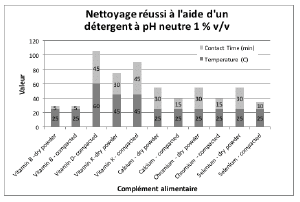
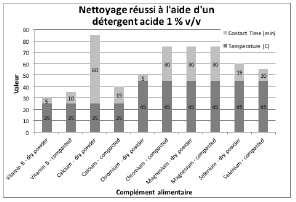
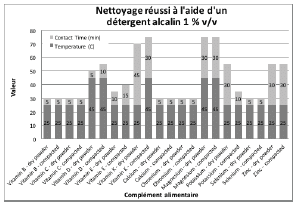
Figures 2 through 5 summarize the results obtained for DI water and the evaluated formulated detergents. Each chart lists the soils that were successfully cleaned by the particular detergent, and includes the temperature and contact time required to achieve the acceptable cleaning results.
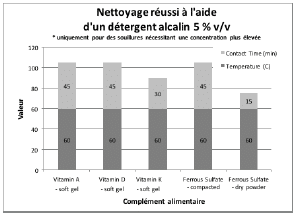
Figure 6 summarizes the group of soils that were not successfully cleaned by DI water or any of the detergents at the desired 1% v/v concentration, even at the highest temperature and contact time parameters of the test. For these soils, a higher detergent concentration of 5 % v/v was evaluated. The alkaline detergent was the only formulation that achieved the acceptable cleaning results for most of the soils tested. Vitamin E soft gel caplet was not successfully cleaned with the DI water, 1% v/v or 5% v/v cleaning agents evaluated in this study. However, vitamin E soft gel caplet was successfully cleaned with a two-product approach consisting of a formulated alkaline detergent at 1% v/v plus a detergent additive containing hydrogen peroxide at 1% v/v, for 30 minutes at 60°C. If vitamin E soft gel caplet is considered the worst case of this cleaning evaluation, then this two-product method should also be effective for all the other products evaluated.
Additional evaluations could be performed to confirm a single cleaning procedure for all vitamins and minerals.
Figure 2 : Summary of cleaning parameters obtained for supplements successfully cleaned with DI water only. The figure denotes the minimum temperature and contact time required to achieve cleanliness of the test coupons.
Figure 3 : Summary of cleaning parameters obtained for supplements successfully cleaned with neutral detergent. The figure shows the temperature and minimum contact time required to obtain the cleanliness of the test coupons.
Figure 4: Summary of cleaning parameters obtained for supplements successfully cleaned with an acid detergent. Chart denotes the minimum temperature and contact time required to achieve cleanliness of the test coupons.
Figure 5: Summary of cleaning parameters obtained for supplements successfully cleaned with an alkaline detergent. Chart denotes the minimum temperature and contact time required to achieve cleanliness of the test coupons.
Figure 6: Summary of cleaning parameters obtained for supplements that required a higher concentration of alkaline detergent. Chart denotes the minimum temperature and contact time required to achieve cleanliness of the test coupons.
Discussion and cleaning recommendations for “worst case” products with vitamins and minerals
For vitamins, solubility information indicated that vitamin B had the highest solubility in water and was successfully cleaned with deionized water and detergents (neutral, acidic, or alkaline). The other vitamins were partially or almost insoluble in water and required a detergent formulated for cleaning. Vitamin D3 had the lowest recommended daily dose, at 5-10mcg, and was the most toxic active substance in rats and mice with an 50-5mg / kg DL50. Soft capsules of liquid or gel required the same cleaning time or a longer cleaning time than the dry residues to be removed. Cleanability studies have shown that the most difficult vitamins to clean are the gel form of vitamins A, D, E and K; vitamins K and D were the dry forms most difficult to clean. The soft capsules of vitamin E required a combination of two detergents and were the most difficult to clean samples evaluated in this study. For minerals, the solubility information indicated that ferrous sulfate, potassium gluconate and zinc gluconate were soluble in water, but in the final dosage forms none were successfully cleaned with de-ionized water. Selenium is the most toxic substance in this group, based on an oral LD50 of 3-7 mg/kg in rats and mice. Ferrous sulfate was the hardest to clean mineral as determined by our cleanability studies, in dry and compacted powder, requiring 5% v/v of the alkaline detergent. Magnesium citrate was the second hardest to clean requiring a 1% v/v alkaline detergent to successfully remove the product. Based on the results obtained in this evaluation, several options may be recommended for grouping. For simplification purposes we are assuming that the products evaluated are manufactured on the same equipment; however this may not be the actual case.
Use of soft vitamin E capsules as the most difficult stain to clean
Soft capsules of vitamin E were the only products requiring the combination of two detergents. Overall, they have been the most difficult stain to clean, so one of the options is to clean all other products using the same cleaning procedure that is recommended for soft vitamin E capsules.
Validation can be performed for vitamin E soft gel caplet, with the acceptable residual value being based on the limits for the most toxic active in the group, selenium. A preliminary evaluation may be necessary to confirm that vitamin E soft gel caplet can be successfully cleaned down to the pre-set limits. If this is not feasible, then two separate validations could be conducted using the cleaning procedure recommended for vitamin E soft gel caplet. One validation is conducted for vitamin E soft gel caplet with cleaning limits based on the toxicity of the next most toxic active. The other validation is conducted for selenium based on the toxicity limits for selenium. The combination of these two studies would constitute the validation of the worst cases within the group in terms of hardest-to-clean and most toxic soils.
Using vitamin E soft gel caplet, vitamin A soft gel caplet, vitamin D soft gel caplet, and compacted ferrous sulfate as representative hardest-to-clean soils
1. Since vitamin E soft gel caplet was the only product requiring a two-detergent combination, a validation study could be performed in which the recommended cleaning procedure and the acceptable residual value are based on vitamin E soft gel caplet only. This will constitute a “one-product” matrix and will be applicable only for cleaning vitamin E soft gel caplet.
2. The second product matrix includes all remaining products and is based on the cleaning procedure recommended for vitamin A soft gel caplet vitamin D soft gel caplet and compacted ferrous sulfate, which follow the same procedure. A validation can be performed for one of these soils, with the acceptable residual value being based on the limits obtained for selenium, or two separate validations studies could be conducted following the cleaning procedure recommended for vitamin A soft gel caplet, vitamin D soft gel caplet and compacted ferrous sulfate. One validation is conducted for any of the aforementioned soils with cleaning limits based on the toxicity of the next most toxic active. The other validation is conducted for selenium based on the toxicity limits for selenium.
Developing a single cleaning procedure for a number of products that are manufactured in the same equipment train using laboratory studies can allow the use of scientifically justified grouping strategies. Grouping strategies can significantly reduce the extent of cleaning validation protocols. A reduction in cleaning validation protocols can save time, resources and money without compromising quality.

Elizabeth RIVERA – Steris
frederic_bar@steris.com
References
(1) Code of Federal Regulations, 21CFR Part 211.67a “Equipment cleaning and maintenance”.
(2) Code of Federal Regulations, 21CFR part 111.25 “Equipment and Utensils”.
(3) The Merck Index (Whitehouse Station, NJ) Twelfth Edition, 1996.
(4) USP 33 NF 28, October 1, 2010 – February 1, 2011.
(5) Hall, W.A. 1997. Cleaning for Bulk Pharmaceuticals Chemicals in Validation of Bulk Pharmaceutical Chemicals; edited by D. Harpaz and I.R. Barry. Buffalo Grove, IL., USA: Interpharm Press. PP 335-370.
(6) STERIS Corporation, Technical Tip #3031, Residue Limits for Cleaning Agents (2003).
(7) Kramer, H.J. et al., Conversion Factors Estimating Indicative Chronic No-Observed-Adverse-Effect Levels from Short-Term Toxicity Data, Regulatory Toxicology and Pharmacology, vol. 23, 249-255 (1996).
(8) Layton, D.B. et al., Deriving Allowable Daily Intakes for Systemic Toxicants Lacking Chronic Toxicity Data, Regulatory Toxicology and Pharmacology, vol. 7, 96-112(1987).
(9) LeBlanc, D.A. 2000. Validated Cleaning Technologies for Pharmaceutical Manufacturing; Englewood, CO., USA: Interpharm Press. PP 121-140.
(10) Dolan, D.G., B.D. Naumann, E.V. Sargent, A. Maier and M.L. Dourson. Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regul. Toxicol. Pharmacol.43, 1-9 (2005).
(11) ISPE Baseline Guide, Risk-Based Manufacture of Pharmaceutical Products, pp. 33 -46 (2010).
(12) EMA/CHMP/CVMP/SWP/169430/2012 – Final November 2014, Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities.
(13) Hall, W.A. 2009. Residue Group Strategies, Chapter 13 in Cleaning and Cleaning Validation, Volume 1 by Paul Pluta, PDA and Davis Healthcare International Publishing LLC, p 265-279.
(14) Dietary Reference Intakes: Vitamins: The National Academics, 2001.
(15) Recommended Dietary Allowances, 10th Edition, 1989, by the Subcommittee on the Tenth Edition of the Recommended Dietary Allowances, Food and Nutrition Board, Commission on Life Sciences National Research Council, pages 284-285.
(16) Kamm, J.J. 1984. Preclinical and Clinical Toxicology of Selected Retinoids, in Sporn, M.B. et al. ed. The Retinoids, Vol. 2, New York, Academic Press, pp. 287-326.
(17) Herold, M., 1975. Toxicology of Vitamin A Acid. Acta Dermato-Venereologica, Vol. 74, Supplementum, pp. 29-32.
(18) Fogarty, P. 2002. In-vivo High Throughput Toxicology Screening Method, U.S. Patent 6,365,129; Washington, D.C.: United States Patent and Trademark Office.
(19) “Safety (MSDS) data for ascorbic acid”. Oxford University. 2010-09-03. http://physchem.ox.ac.uk/MSDS/AS/ascorbic_acid.html. Retrieved 2011-08-22
(20) Sanseverino, J. 2005. Ascorbic Acid, in Wexler, P. et al. ed. Encyclopedia of Toxicology, 2nd ed., Oxford: Elsevier, p.182-184.
(21) Material Safety Data Sheet for DSM Dry Vitamin D3 100 SD/S 5005043 Version 1.3 dated 07/07/2010
(22) Final Report on the Safety Assessment of Tocopherol, Tocopheryl Acetate, Tocopheryl Linoleate, Tocopheryl Linoleate/Oleate, Tocopheryl Nicotinate, Tocopheryl Succinate, Dioleyl, Tocopheryl Methylsilanol, Potassium Ascorbyl Tocopheryl Phosphate, and Tocophersolan. International Journal of Toxicology November 2002 21 (3 Suppl): 51-116.
(23) Krasavage, WJ and Terhaar, CJ. 1977 d-alpha-Tocopheryl poly(ethylene glycol) 1000 succinate. Acute Toxicity, Subchronic Feedings, Reproduction, and Teratologic Studies in the Rat, International Journal of Toxicology. Vol. 25, (2) pp. 273-278.
(24) Molitor, H. and Robinson, H.J. 1940 Oral and Parenteral Toxicity of Vitamin K1, phthicol and 2-methyl-1,4-naphthoquinone. Proceedings of the Society for Experimental Biology and Medicine, 43,125-128.
(25) “Safety (MSDS) data for calcium carbonate”. Sciencelab.com 11/01/2010, http://www.sciencelab.com/msds.php?msdsld=9927119, Retrieved Aug.22, 2011.
(26) Recommended Dietary Allowances, 10th Edition, 1989, by the Subcommittee on the Tenth Edition of the Recommended Dietary Allowances, Food and Nutrition Board, Commission on Life Sciences National Research Council, pages 284-285.
(27) Madinaveitia, JL. 1965. Ferrocenes as Haematinics British Journal of Pharmacology Vol. 24, pp. 352-359.
(28) Yeary, R.A. et al. 1966 Acute Toxicity of Drugs in Newborn Animals, Journal of Pediatrics Vol. 69 (4) pp. 663-667.
(29) Vuignier, BI et al. 1989 Effect of magnesium Citrate and Clidinium Bromide on the Excretion of Activated Charcoal in Normal Subjects DICP, The Annals of Pharmacotherapy Vol. 23 pp. 26-29.
(30) Zwerling, H. 1991 Hypermanesemia-Induced Hypotension and Hypoventilation. Journal of the American Medical Association Vol. 266 (17) pp. 2374-2375.
(31) Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate, 2004, by the Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, http://www.nap.edu/catalog/10925.html, pp. 234-235.
(32) Kiso to Rinsho, Clinical Report, Vol. 20 (9) pp. 217-226.
(33) Mizuta, T. 1981. General Pharmacological Study of Citrate-Citric Acid Combination (Uralyt-U®) Oyo Yakuri: Pharmacometrics 1981 Vol. 21, pp. 715.
(34) EMEA/MRL/249/97-Final October 1997, The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit, EMEA/MRL/249/97-Final October 1997, Committee for Veterinary Medicinal Products – Potassium and Sodium Salts of Selenium Summary Report.
(35) Wang, B et al. 2006 Acute Toxicity of Nano- and Micro-Scale Zinc Powder in Healthy Sdult Mice, Toxicology Letters, Vol. 161, pp. 115-123.
(36) Hadziselimovic, D. et.al., Critical Cleaning of Carbomer Containing Products; Journal of GXP Compliance, Vol. 16, No. 3, pp 1-6 (2012).
(37) Hadziselimovic, D., et.al. 2015. Cleaning Acrylic Polymer Enteric Coatings. The International Waterborne, High Solids and Powder Coatings Symposium, 2nd Ed, edited by M. Heusser and S. Mendon, Raleigh, NC, Lulu Press Inc., pp 367-380.
Par Elizabeth RIVERA & Paul LOPOLITO – Steris