A3P Aseptic processing
Annex 1, microbiology, Single use
Luogo
Enterprise hotel, Milano
Data
10 e 11 maggio 2023
Save The Date !

Formato
Conferenze, esposizione, workshop dei partner
Condividere
Siamo lieti di annunciare l’evento A3P Aseptic Processing che si terrà a Milano il 10 e 11 maggio. L’evento sarà dedicato ai temi chiave dell’Annex 1, dell’utilizzo di sistemi monouso e microbiologia. Esperti del settore si confronteranno per discutere delle ultime novità in materia di produzione aseptica e condivideranno le loro esperienze sul campo. L’evento rappresenta un’opportunità unica per tutti coloro che lavorano nel settore farmaceutico e biotecnologico per approfondire le proprie conoscenze e mettersi in contatto con altri professionisti del settore.
Mercoledì 10 maggio
Giovedì 11 maggio

OF CAPE COD
INTERNATIONAL
Modulo di registrazione
A3P Aseptic processing
Una volta compilato e inviato il modulo, si riceverà una copia via e-mail.
Quota di iscrizione: : 500€ senza IVA (IVA 20%)
Mappa dell’esposizione
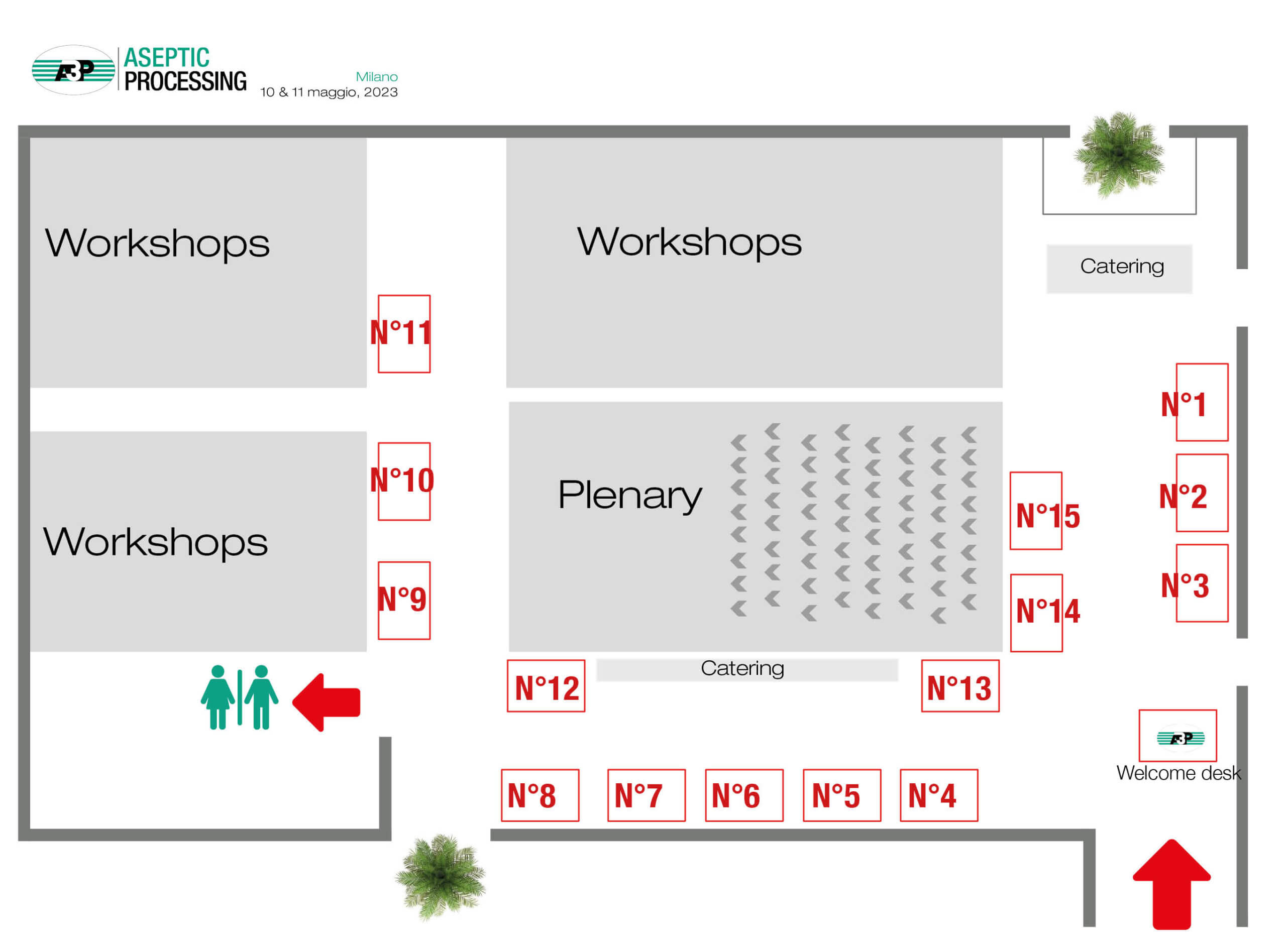
| Company | Desk N° | Société | Desk N° | |
| LABORATOIRE HUCKERT’S INTERNATIONAL ITALY | 1 | BD | 9 | |
| MERCK | 2 | CONTEC | 10 | |
| BURKERT ITALIA | 3 | AM INSTRUMENTS | 11 | |
| IMA S.p.A. INDUSTRIA MACCHINE AUTOMATICHE | 4 | FPS FOOD AND PHARMA SYSTEMS srl | 12 | |
| STERIS – Partner | 5 | RAPID MICRO BIOSYSTEMS | 13 | |
| GROUPE ICARE | 6 | ASSOCIATES OF CAPE COD INTERNATIONAL – Partner | 14 | |
| NOVATEK INTERNATIONAL – Partner | 7 | VEOLIA WATER – SIEVERS INSTRUMENTS | 15 | |
| JCE BIOTECHNOLOGY | 8 |
Servizi inclusi nel desk espositivo
- Uno spazio espositivo dedicato su cui installare i vostri prodotti e strumenti di comunicazione: roll-up, prodotti, … Un’attrezzatura di base che comprende un tavolo di 180×80 cm, 2 sedie e 1 presa elettrica.
- Pause e pasti come indicato nel programma
- Wifi illimitato
- Un badge che dà libero accesso all’intero programma tecnico e scientifico

Contatto
Per qualsiasi domanda si può contattare Natalina Semedo :
Telefono : +33 (0)4 37 28 30 52 / +33 (0)7 58 69 26 30
Email : nsemedo@a3pservices.com
Accessibilità



