Summary
- Limit between GMP Part I/ Part II : Applicability to biological products
- Moving One Unit Operation At a Time Toward Continuous Biomanufacturing
- « Close Collaboration Maximizes Value of Engineered Solutions and Saves Time in Start-Up »
- Improving Single Use Bioreactor Design and Process Development. New Research Towards Intensifying Seed- Train & Scale-up Methods Using 5:1 Turn- Down
- Qualification approach for the validation of real-word shipping in single-use systems
- Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in BioBLU 0.3c Single-Use Bioreactors
- Single Use & Stainless Steel: complementarity or fight?
- Low Endotoxin Recovery (LER) is today one of authorities serious concerns regarding pyrogen testing
Mesenchymal stem cells (MSCs) are attractive candidates for therapeutic applications, especially in the field of regenerative medicine[1] because – in contrast to embryonic stem cells – they do not pose ethical issues, they can be isolated from various tissue sources, and they reduce the risk of rejection reactions. The doses of human MSCs (hMSCs) needed for clinical trials are estimated at between one and 200 million cells per patient, depending on the disease being tackled[2].
One of the most important challenges in providing hMSCs for curative use is the production of large quantities of cells in a robust manner.
Indeed, whatever the tissue source, the number of hMSCs extracted is very low, and not sufficient for clinical use; hence the hMSCs have to be expanded following isolation. Besides providing the needed cell quantities, hMSC production must also comply with the manufacturing process regulations required of a fully controlled production system. hMSC expansion in stirred-tank bioreactors can be monitored and is scalable, and hence can fulfil these requirements from experimental quantities to production. In the study presented here, we used an Eppendorf DASbox Mini Bioreactor System equipped with BioBLU 0.3 Single-Use Vessels to culture adherent multipotent stem cells on microcarriers, and reached a clinically relevant number of cells.
Material and Methods
Culture of human bone marrow-derived mesenchymal stem cells (hMSCs-BM)
We cultured hMSCs-BM (Lonza) at 37°C in MSCGM Mesenchymal Stem Cell Growth BulletKit Medium (Lonza), including both the basal medium and the necessary supplements. The cells were first expanded in Eppendorf Cell Culture Flasks. At passage six, we harvested the cells to be used for three-dimensional microcarrier culture in a stirred-tank bioreactor.
We processed the experiments in parallel in a 4-fold Eppendorf DASbox Mini Bioreactor System for cell culture, equipped with Eppendorf BioBLU 0.3c Single-Use Vessels. We inoculated the cultures with hMSCs-BM combined with Cytodex type 1 (GE Healthcare Bio-Sciences) or Cytodex type 3 (Sigma-Aldrich) microcarriers. The initial number of cells per bioreactor was 6 x 106 cells, which corresponds to a cell density of 9,700 cells/cm2 for Cytodex type 1 and 11,000 cells/cm2 for Cytodex type 3.
The initial working volume was 100 mL. To promote the initial cell adhesion, we did not agitate the culture for 24 hours. After this attachment period, we manually adjusted the culture volume to 200 mL, and set the agitation speed to 60 rpm for the entire proliferation phase. The pH of the growth medium was controlled at 7.6 by automatic addition of COcm2 in the vessel headspace. The dissolved oxygen (DO) level was maintained at 40 % by delivering gas (N2, air, and O2) into the medium with the gas flow set at 0.1 sL/h. To maintain the culture until its maximum yield, we exchanged 50 % of the culture medium after 6 and 8 days of culture. After 9 days, up to 70 % of the medium was replaced almost daily. During those refreshment steps, agitation was stopped to let the microcarriers sediment.
The cells were cultivated for 20 days on Cytodex type 1 and for 27 days on Cytodex type 3 microcarriers.
Analysis of cell viability, cell counting, and metabolite measurement
During the proliferation phase, we regularly analyzed the cell viability on the microcarriers by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) coloration.
To evaluate cell growth, we collected samples of the cell/microcarrier suspensions, detached the cells from microcarriers and determined the cell number using the CASY Cell Counter and Analyzer, model TT (Omni Life Science). We used the supernatants collected during cell counting to quantify glucose and lactate concentrations, using the Glucose RTU Kit (bioMérieux) and L-Lactate Assay Kit (Abcam), respectively.
hMSC-BM differentiation
We detached hMSCs-BM from microcarriers by trypsin treatment, and seeded them into BD BioCoat Fibronectin Cellware, 24-well plates (Corning).
We induced differentiation of the hMSCs into mature osteoblasts and chondrocytes, respectively by replacing the expansion medium with hMSC Osteogenic Differentiation BulletKit Medium and Chondrogenic Differentiation BulletKit Medium (Lonza), respectively.
At days 14 and 21 we assessed the bone cell mineralization using the OsteoImage Mineralization Assay (Lonza). Moreover, at day 21 we stained calcium deposits with an anthraquinone dye (Alizarin Red S Staining Kit, ScienCell). At day 14, we detected proteoglycans secreted by chondrocytes by Alcian Blue staining (Sigma-Aldrich).
Results
hMSC-BM expansion on microcarriers in BioBLU 0.3c Single-Use Vessels
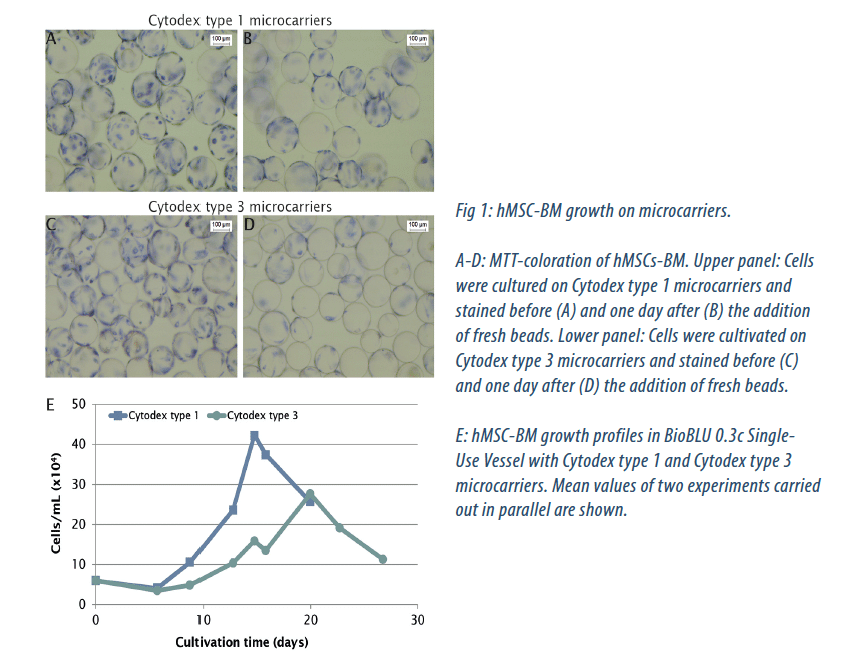
To investigate the suitability of BioBLU 0.3c Single-Use Vessels for the scalable production of multipotent stem cells, we cultured hMSCs-BM in parallel on two microcarriers types. Cytodex type 1 microcarriers are based on a dextran matrix covered with positively charged groups, while a layer of denatured collagen is covalently bound on the Cytodex type 3 dextran surface. One possibility to expand cells in a microcarrier culture is the use of a technique called bead-to-bead transfer. By adding fresh microcarriers into the existing culture, cells can switch from one carrier to another, and start to grow on the empty beads [2, 3, 4].
This allowed us to avoid subculturing techniques traditionally used with adherent cells. When stained cells were visible on all beads (detected by MTT coloration) we added fresh microcarriers to offer additional growth surface (Fig. 1A-D). We obtained the best proliferation rate on Cytodex type 1 microcarriers (Fig. 1E). The cell number increased 17.5 fold to a maximum cell density of 1 x 108 cells/bioreactor at day 14, corresponding to 4 x 105 cells/mL. On Cytodex type 3 microcarriers, 20 days of culture were needed to reach a maximum cell number of 7 x 107 cells per bioreactor, which is 11.5-fold higher than the initial seeded quantity and corresponds to 2.5 x 105 cells/mL. Glucose monitoring revealed a rapid nutrient consumption of the hMSC-BM culture on both microcarrier types.
As a result of glucose consumption and feeding, the glucose concentration varied in a characteristic pattern, but never dropped below 0.1 g/L (not shown). As by-products of their glucose metabolism, the cells produce metabolites, such as lactate and ammonia, which accumulate in the medium (not shown). High lactate concentrations can affect hMSCs growth as well as cell morphology. We maintained the lactate concentration below 25 nM to ensure that a concentration of 35.4 nM, which was found to inhibit growth[5], was never reached.
Multipotency analysis of hMSCs-BM
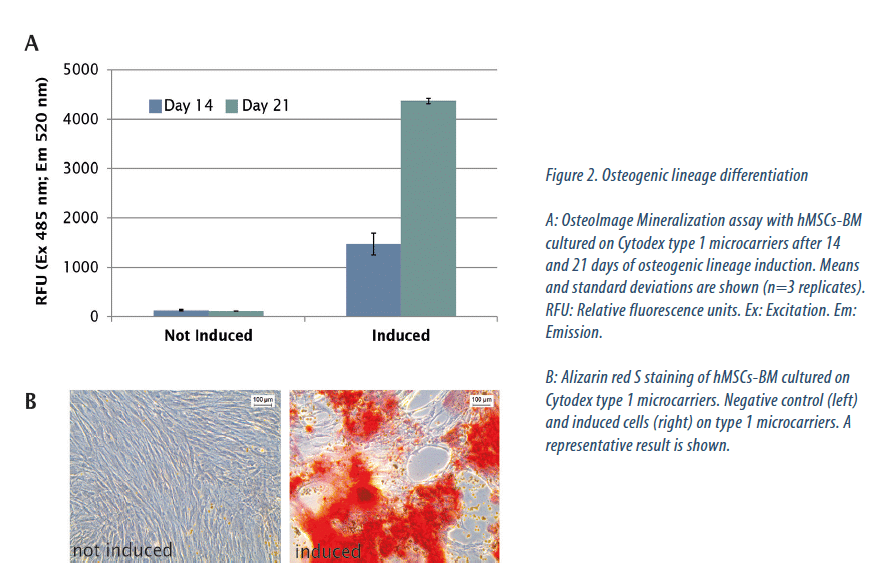
To confirm that hMSCs-BM cultured in BioBLU 0.3c Single-Use Vessels retained their differentiation capacity, we used them for in vitro osteogenic and chondrogenic differentiation assays. We obtained equivalent results with cells cultured on Cytodex type 1 and 3 microcarriers. Osteogenic differentiation is divided into three stages: cell proliferation (from day 1 to 4), extracellular matrix maturation (from day 5 to 14), and finally, matrix mineralization (from day 14 to 28). This last step is characterized by high expression of osteocalcin and osteopontin, followed by calcium and phosphate deposition[6]. Bone is composed of the organic protein collagen and the inorganic mineral hydroxyapatite. Bone mineralization can be quantified by specific staining of the hydroxyapatite portion. Bone mineralization was detectable already after 14 days of induction and was clearly increased at day 21. Hydroxyapatite was only detected on induced cells (Fig. 2A). After 21 days of osteogenic lineage induction, calcium deposits, indicating the matrix mineralization phase, were furthermore detected by Alizarin Red S staining on induced hMSCs-BM, while we observed no deposition in the not-induced negative control (Fig. 2B). Next we assessed chondrogenic differentiation, which is a complex, multistage process characterized by the production of cartilage-specific molecules such as type II collagen and proteoglycans. Detectable by Alcian Blue staining, proteoglycans are considered to be a marker of cell chrondrogenesis. After 14 days of chondrogenic lineage induction, we detected strong cartilage proteoglycan synthesis, while the level stayed low in the negative control (Fig. 3).
Conclusion
In summary, our results demonstrate that the DASbox Mini Bioreactor System equipped with BioBLU 0.3c Single-Use Vessels is suitable to expand multipotent stem cells in a safe and controlled manner.

Vincent DUFEY, Aurélie TACHENY, Muriel ART, Ulrike BECKEN & Françoise DE LONGUEVILLE – EPPENDORF
becken.u@eppendorf.com
Share article
References
[1] Wang S, Qu X, Chunhua Zhao R. Clinical applications of mesenchymal stem cells. Journal of Hematology and Oncology Review 2012; 5:1-9.
[2] Jung S, Panchalingam K M, Wuerth R D, Rosenberg L, Behie L A. Large-scale production of human mesenchymal stem cells for clinical applications. Biotechnology and Applied Biochemistry 2012; 59:106-120.
[3] Wang Y, Ouyang F. Bead-to-bead transfer of Vero cells in microcarrier culture. Cytotechnology 1999; 31:221-224.
[4] Sart S, Schneider Y-J, Agathos S N. Ear mesenchymal stem cells: An efficient adult multipotent cell population fit for rapid and scalable expansion. Journal of Biotechnology 2009; 139:291-299.
[5] Schop D, Janssen F W, van Rijn L D S, Fernandes H, Bloen R M, de Bruijn J D ven Dijkhuizen-Radersma R. Growth, Metabolism and Growth Inhibitors of Mesenchymal Stem Cells. Tissue Engineering 2009; 15:1877-1885.
[6] Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors to osteoblasts in vitro. Tissue Engineering 2007; 13: 2311-2320.
[7] Schop D, Janssen F W, van Rijn L D S, Fernandes H, Bloen R M, de Bruijn J D ven Dijkhuizen-Radersma R. Growth, Metabolism and Growth Inhibitors of Mesenchymal Stem Cells. Tissue Engineering 2009; 15:1877-1885.
Glossary
Ex: Excitation
Em: Emission
hMSC: human mesenchymal stem cell
hMSC-BM: bone marrow-derived human mesenchymal stem cell
MSC: mesenchymal stem cell
MTT: 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
RFU: Relative fluorescence units