MICROBIOLOGY
Rapid microbiology - Alternative Methods - Regulatory
LOCATION
Novotel Madrid City Las Ventas
Madrid, Spain

Format
Conferences, Workshops, Exhibition, Networking
Share
Microbiological control in the pharmaceutical industry is one of the fundamental pillars to have safe medicines in the market.
This year, your reference association and the A3P Spain Committee invite you to participate in the presential event within the framework of microbiological quality control:
→ Incorporate rapid microbiology into pharmaceutical production processes increases productivity and safety, allows for earlier detection of any contamination and for fast and effective action.
🎧 simultaneous translation service : Spanish / English
› SPEAKERS
🎧 simultaneous interpretation: English / Spanish

Our partners will offer you 3 sessions of workshops
Cape Cod
of Cape Cod
Cape Cod
Registration Form
Become A3P member and participate in A3P Microbiology event.
Price: 500€ (+membership up to date)
Forum A3P Microbiology – April 7th, 2022 in MADRID
The exhibition is full
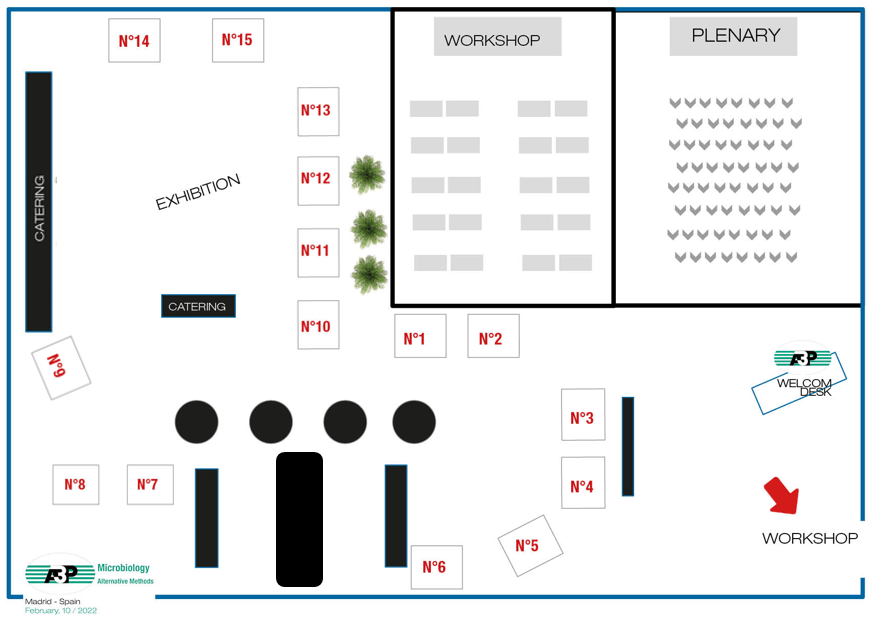
| Company | Stand n° | Company | Stand n° | |
| LOGOS BIOSYSTEMS | 1 | RAPID MICRO BIOSYSTEMS | 9 | |
| INSTRUMENTACIÓN ANALÍTICA | 2 | NOVATEK INTERNATIONAL | 10 | |
| MERCK | 3 | BIOMERIEUX | 11 | |
| TISELAB | 4 | STERIS | 12 | |
| CHARLES RIVER | 5 | ASSOCIATES OF CAPE COD | 13 | |
| BECTON DICKINSON | 6 | CONFARMA | 14 | |
| MESALABS | 7 | BWT | 15 | |
| VELTEK ASSOCIATES Inc. | 8 |
Services included with the Table Top stand
- A dedicated exhibition area where you can install your products and communication tools: umbrella stand, roll-up, display, product, …
A basic equipment including a 180×80 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker - Breaks and meals as indicated in the program
- Illimited Wifi
- A badge that gives you free access to the entire technical and scientific programme.
- The list of participants to the event
Contact
Depending on your requirements and preferences, you can contact Florian Canuto:
Phone number: +33 (0)7 61 55 24 36
Email : fcanuto@a3pservices.com
Accessibility :





