ANNUAL CONGRESS A3P MIDDLE EAST 2025
Quality Risk Management (ICH Q9 R1)/ EU GMP Annex 1 / Digital Transformation & AI / Validation
Locate
Date
February 12 & 13, 2025
Save The Date !

Format
Conferences, workshops and exhibition
Share
The A3P Middle East congress is a premier event in the pharmaceutical industry, bringing together professionals from across the region to discuss the latest advancements in sterile drug manufacturing and quality control.
With a main theme related to Sterile Manufacturing and the Deployment of the New Annex 1 in the Middle East, this first conference in the region promises to be a great opportunity to network with peers and learn from experts in the field.
The conference program includes a variety of sessions, workshops, and panel discussions covering topics such as quality risk management (ICH Q9 R1), EU GMP Annex 1 and contamination control. Attendees will also have the chance to visit the exhibition hall, where they can explore the latest products and services from leading pharmaceutical companies.
Whether you’re a seasoned professional or new to the field, this event is a must-attend for anyone interested in staying up-to-date on the latest trends and technologies in pharmaceutical manufacturing.
Official language of the conferences: English
Wednesday, February 12
Thursday, February 13
Participants will gain insights into the systematic process of vendor selection, emphasizing criteria that align with organization standards and regulatory requirements. A detailed discussion on vendor classification will follow, covering risk-based classification frameworks and their applications in ensuring that resources are allocated effectively across different types of vendors. The workshop will outline the essential documentation for vendor selection, providing clarity on necessary contracts, agreements, and quality assessments to meet compliance standards.
The course will then delve into the fundamentals of vendor audits, explaining the planning, execution, and follow-up required to evaluate vendor compliance with quality standards and regulatory expectations. Additionally, we will examine vendor oversight strategies to maintain ongoing quality and compliance throughout the vendor relationship. Vendor performance analysis will round out the session, focusing on metrics, scorecards, and improvement plans to ensure consistent, high-quality outputs from vendors.
Through a mix of lectures, case studies, and interactive discussions, attendees will leave with a comprehensive toolkit for managing vendors in a way that minimizes risk and maximizes quality, efficiency, and regulatory compliance in pharmaceutical operations.
- The new requirements and expectations of Annex 1 regarding Aseptic Process Simulation (APS)
- Regulatory inspection feedback
- Group discussions around various questions and real-life situations that industry professionals may encounter, such as : determining of the APS strategy (initial and periodic) in the context of campaign-based production, the management of line stoppages (case of technical shutdowns), management of non-compliant APS, units' reconciliation
- Why is it important to define Critical Process Parameters (CCP)
- What are the critical CCPS´s for BFS process
- How to detemine and identify the CCP´s
- Working groups for different BFS products to define the CCP´s in a working group and present the results
Map of the exhibition
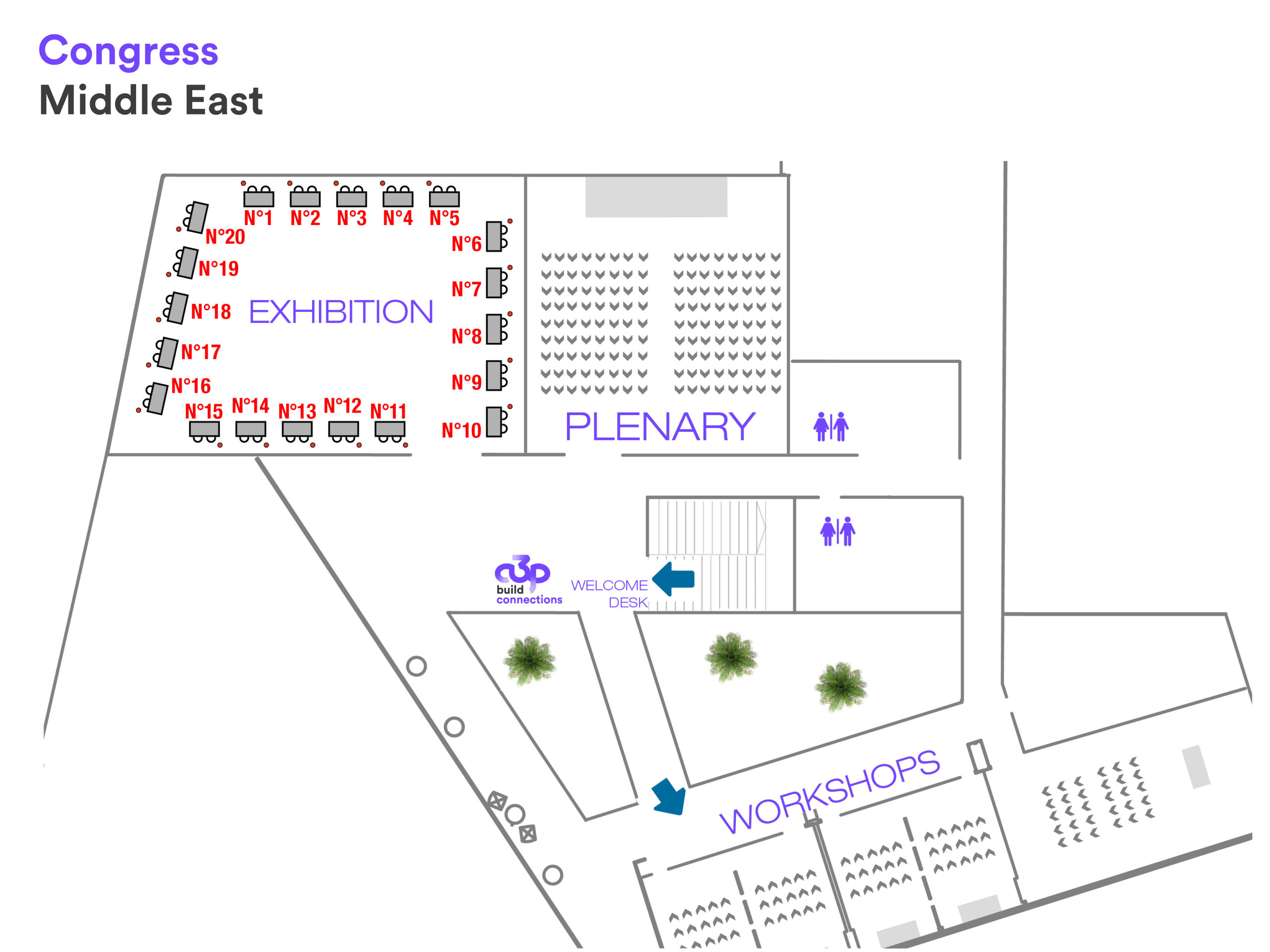
| Company | Stand N° | Société | Stand N° | |
GPS – GULF PHARMACEUTICAL CONSULTANCIES | 1 | K | 11 | |
| SRM TECHNOLOGIES | 2 | L | 12 | |
| ROMMELAG | 3 | M | 13 | |
| D | 4 | N | 14 | |
| PIERCAN | 5 | O | 15 | |
| F | 6 | P | 16 | |
| M-PARTNERS | 7 | Q | 17 | |
| H | 8 | R | 18 | |
| I | 9 | S | 19 | |
| J | 10 | T | 20 |
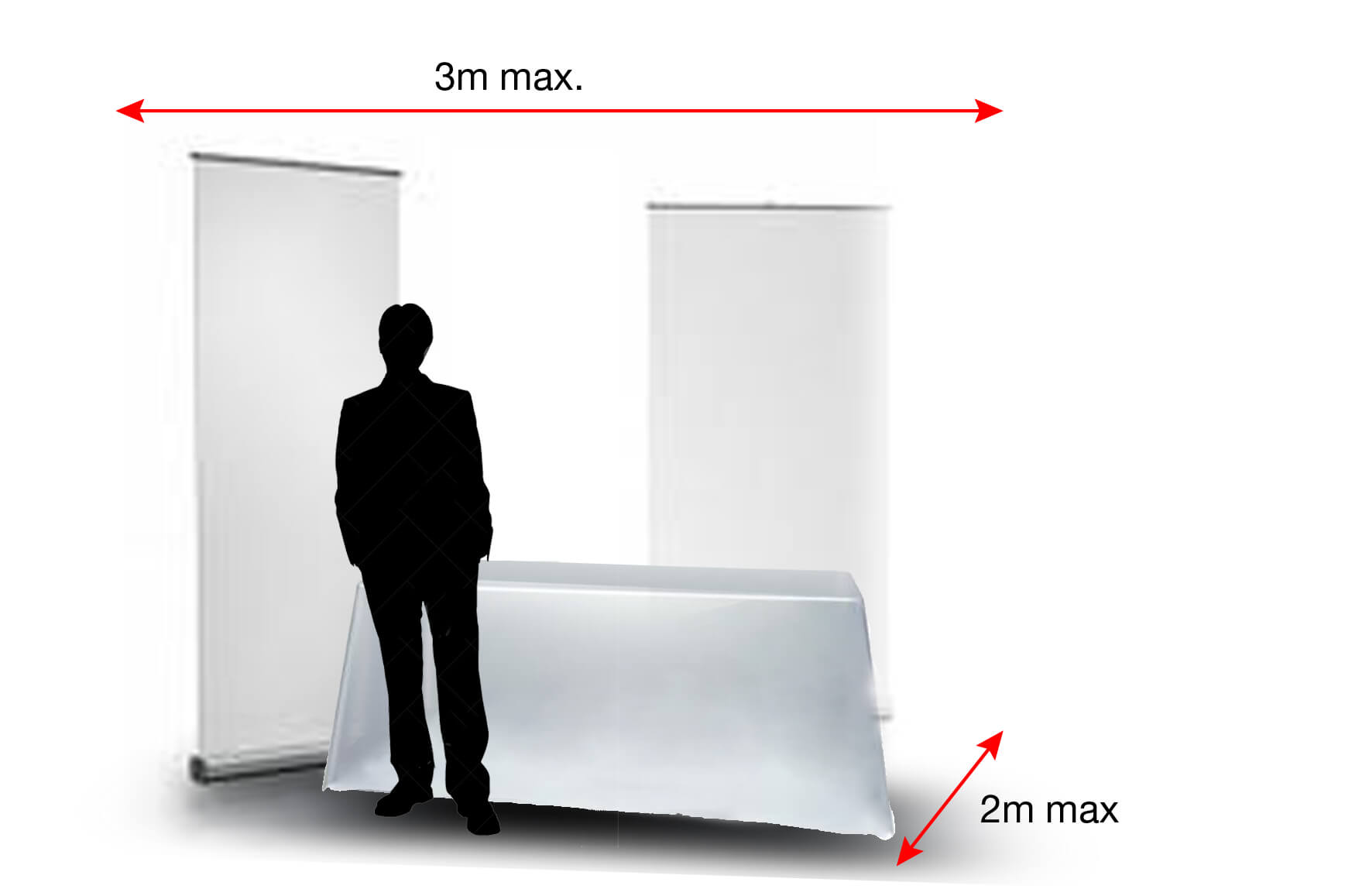
Services included with the Table Top stand
- A dedicated exhibition space on which you can install your products and
communication tools: umbrella stand, roll-up, display stand… delimited by a
structure with back partitions and returns. - A basic equipment including a table 180 x 80 cm, 2 chairs and 1 electrical
connection (16A, 300 kw) without circuit breaker, a row of 2 spotlights, the
loan of a Nespresso coffee machine with pods. - Breaks and meals as indicated in the programme
- Unlimited Wifi
- A badge that gives you free access to the entire technical and scientific
programme - The list of participants to the event
- Possibility to register an additional person at the special “accompanying
person” rate
Exhibitor Form
Congress A3P Middle East 2025 – Jeddah, Saoudi Arabia
Once you have completed this form, you will receive a copy by email
Registration form
Middle East A3P Congress – Jeddah, Saoudi Arabia 2025
Once you have filled in this form, you will receive a copy by email
Registration fee: 1,800 SAR (excl. VAT) per person and for 2 days
For further information please contact Mohamed SAFRI:
Phone: +33 (0)4 37 28 30 40
Email: msafri@a3pservices.com
Accessibility

