Sommaire
- Gene therapy manufacturing comes of age: Commercial-scale manufacturing is imminent. Are gene therapy innovators ready?
- Overcoming obstacles in downstream bioprocessing of AAV based gene therapy products
- Overcoming challenges in the development of lentiviral vector manufacturing platforms
- Are modern scalable bioreactors the Cell Culture Strategy needed for Gene & Cell Therapy success?
- The magnetic power of nanoparticles magnetic cell sorting decontamination of-environments and reusable nanocatalysts
- Development of a new preventive approach to reduce microbial infections with metal oxide nanoparticles
- Maximizing Sterility Assurance: Sterile Hold Time Testing for Sterilized Items Used in Parenteral Drug Manufacturing
Are modern scalable bioreactors the Cell Culture Strategy needed for Gene & Cell Therapy success?
Gene and cell therapies are innovative biological therapeutics developed especially for cancer and inherited monogenetic diseases. They represent an alternative to chronic therapies making a “oneshot” cure for many diseases and conditions a reality. However, process development time and manufacturing costs issues are slowing down the spread of gene and cell therapies.

The standard strategy is to encapsulate the therapeutic gene in a carrier, usually a viral vector, then produce this in cell culture. However, the viral vector production industry still partially uses open and manual processes, while gene and cell therapy require closed, automated, and scalable platforms, which should speed up development and reduce manufacturing costs.
Modern scalable bioreactors allow rapid scale-up from development to production scale. They ensure a high degree of automation and monitoring which reduces costs, manpower, and contamination risks. They can be easily paired with continuous processing steps – like tangential flow filtration – to increase cell density and viability. Many solutions are available on the market for both adherent and suspension cell culture and in disposable formats.
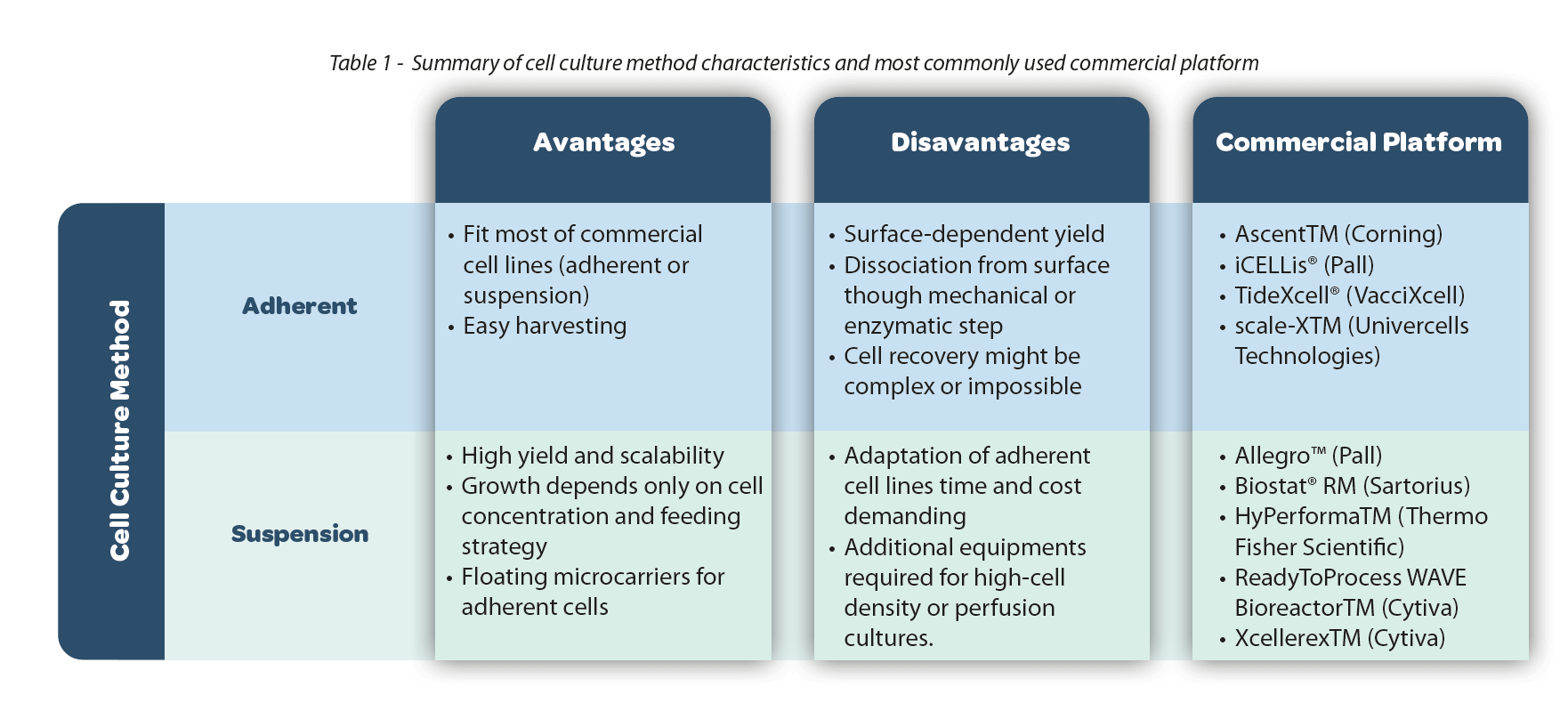
1. Cell culture method: adherent vs suspension
The selection of the bioreactor depends on the cell culture method: adherent, where cells need a solid substrate to grow as a monolayer, or suspension, where the cells can grow free-floating in the medium. Most mammalian and insect cell lines are naturally anchorage-dependent with only a few exceptions, like hematopoietic cells. The yield, footprint and scalability will therefore be limited by the available growth surface. The use of flatware flasks allows easy cell monitoring under an inverted microscope, but periodic subculture steps are required. In addition, dissociation from the substrate, for manipulation or harvesting, requires a mechanical or enzymatic action. The use of microcarriers as a substrate avoids having to adapt adherent cells for suspension but has some disadvantages. Microcarriers are difficult to handle, and their removal requires a dedicated purification step. Moreover, high shear stress and turbulence generated in stirred-tank bioreactors (see next section) might jeopardize adherent cells’ capacity to grow and survive.
Suspension cells float in the medium where growth is limited only by cell concentration and the feeding strategy. Subculture is easier and cell monitoring is possible through daily sampling or using a cell monitoring probe, available for most bioreactor setups. Even if no dissociation step is needed, some cell lines could present a tendency to aggregate requiring the use of cell strainers or the addition of anti-clumping reagents which are often incompatible with the transfection reagent. Most adherent cell lines can be adapted to grow in suspension, but adaptation can be an expensive and time-consuming process. The market provides some ready-to-use adapted suspension cells that often come with royalties and/or license fees.
2. Scalable and high-capacity cell and gene therapy manufacturing
Gene and cell therapy manufacturing presents several challenges regarding capacity, footprint, and costs. As a result, there is a need for flexible technologies that can accommodate different processes and scales. Currently, the technology landscape for cell and gene therapy production can be classified into adherent (e.g., flatware and packed bed reactors [PBRs]) and suspension technologies (e.g., stirred tank reactors [STRs]) as described above. The scale-X™ bioreactor systems offer scalable bioprocessing solutions for high demand applications. The technology comprises a fabric matrix that offers a large surface area packed into a small bioreactor volume, which embodies the concept of process intensification and significantly reduces the process volume, footprint, and the shear stress on the cells, all while drastically increasing the capacity. It features a unique structured fixed bed, enabling homogeneous cell distribution and media flow. This novel design promotes high cell densities without aggregation while maintaining a low shear stress, resulting in higher specific cell productivity (2- to 4-fold) and product quality (functional/physical titers) for both adherent and suspension cell lines. The cell immobilization inside the scale-X bioreactor fixed-bed also allows for easy implementation of perfusion processes, in-line product concentration and reduction of impurities for a low-volume, concentrated bulk product harvest. The scalable portfolio offer solutions from research (2.4 m² of growth surface) to R&D (10 and 30 m² of growth surface) and manufacturing-scale (200 and 600 m² of growth surface), enabling to reach high-capacity production of cell and gene therapies for suspension and adherent cell lines.
3. Conclusion
Gene and cell therapies based on viral vectors are promising life changing treatments, but their success requires a change in paradigm from the traditional biopharma industry approach, starting with cell culture strategy and scalability. Modern scalable bioreactors can fast track the design of new, closed, secure, and automated platforms for the development and production of gene and cell therapy both in adherent and suspension cell culture. Adherent-based bioreactors can host most of the cell lines on the market, with easy perfusion and harvesting, but scalability is surfacedependent. Suspension bioreactors allow high yield and better scalability, but they present more challenges when implementing perfusion and not all commercially available cell types can be adapted to suspension conditions. New structured fixed-bed of scale-X bioreactor portfolio offer manufacturing solutions to limitations in capacity, reliability and scalability with an easy implementation of perfusion process for both adherent and suspension cell lines and can address the limitations of traditional methods used in cell and gene therapy manufacturing.
Partager l’article