Sommaire
- Gene therapy manufacturing comes of age: Commercial-scale manufacturing is imminent. Are gene therapy innovators ready?
- Overcoming obstacles in downstream bioprocessing of AAV based gene therapy products
- Overcoming challenges in the development of lentiviral vector manufacturing platforms
- Are modern scalable bioreactors the Cell Culture Strategy needed for Gene & Cell Therapy success?
- The magnetic power of nanoparticles magnetic cell sorting decontamination of-environments and reusable nanocatalysts
- Development of a new preventive approach to reduce microbial infections with metal oxide nanoparticles
- Maximizing Sterility Assurance: Sterile Hold Time Testing for Sterilized Items Used in Parenteral Drug Manufacturing
Development of a new preventive approach to reduce microbial infections with metal oxide nanoparticles
The proliferation of multidrug-resistant pathogenic bacteria is considered a real health challenge around the world[1]. Infectious diseases associated with biofilms represent more than 80% of microbial infections in the organism, leading to an increase in patient morbidity and hospital/medical care [2,3].
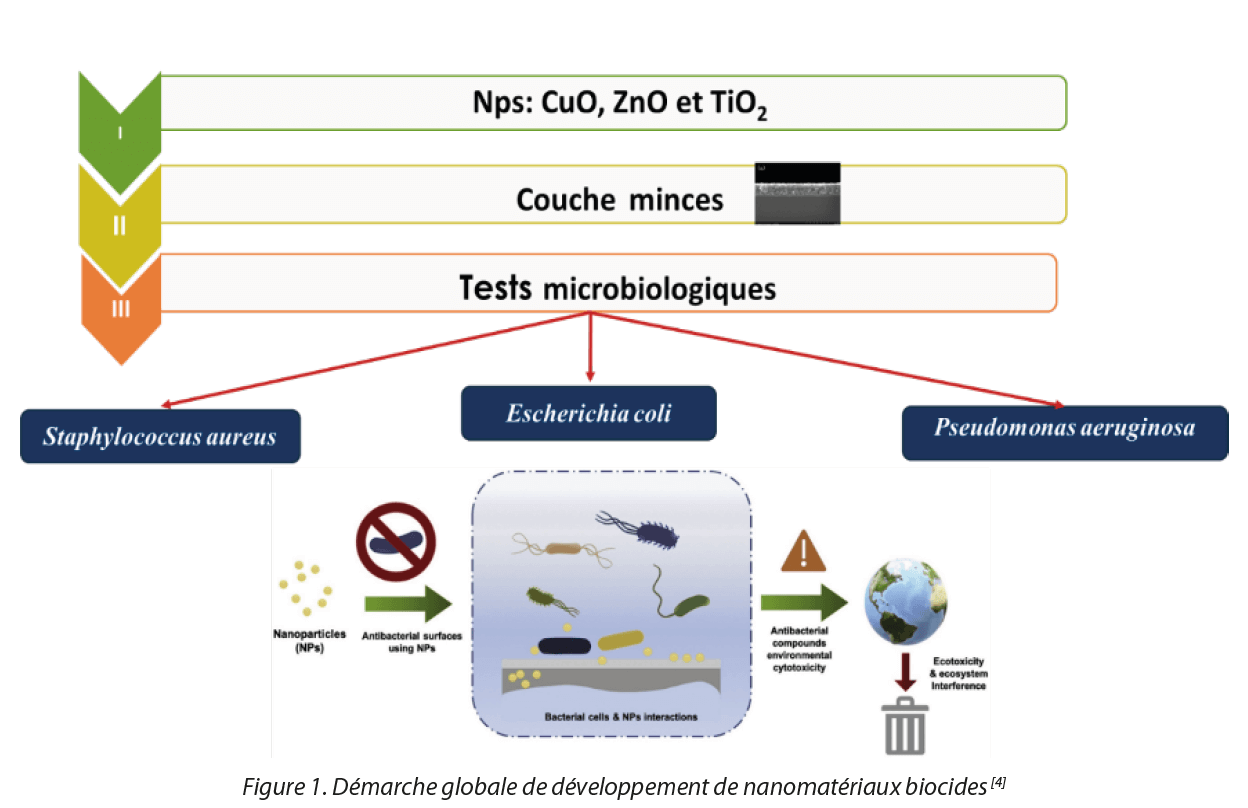
Figure 1. Global initiative for the development of biocidal nanomaterials[4]
Progress in the field of nanobiotechnologies, in particular the capacity to prepare nanomaterials using metal oxides of specific size and shape, is likely to lead to the development of new antibacterial agents. The EBiocide project was born out of the desire to develop nanomaterials with biocidal activities for thin film applications, which would provide a response to problems of nosocomial infections and those of other biomedical and industrial applications. These nanomaterials are considered promising agents against the spread of microorganisms that are resistant to traditional antibiotics due to their broad spectrum antimicrobial properties. The availability of a large specific surface area and their size give these materials unique antimicrobial mechanisms comprising mainly: the generation of reactive oxygen species (ROS); the release of metal ions and destruction of the cell membrane by direct contact[4]. These multiple mechanisms, of physical and chemical origin, are therefore thought to reduce the appearance of resistance on the part of microorganisms.
Initial studies focused on the synthesis of metal oxide nanoparticles (ZnO, CuO) in the form of colloids and in the form of thin films in order to evaluate their antibacterial activities for industrial applications. We carried out cytotoxicity tests of NPs on dermal fibroblast cells. The initial results did not show a toxic effect on cells and other tests are in progress. We carried out mechanical resistance tests on thin films with heat treatment at 500 °C. Resistance was good, but this must be done under actual formulation conditions. The purpose is the use of these nanomaterials for the decontamination of surfaces particularly in the hospital setting and other industrial applications. Figure 1 presents a summary diagram of the overall approach adopted for the development of these biocidal nanomaterials.
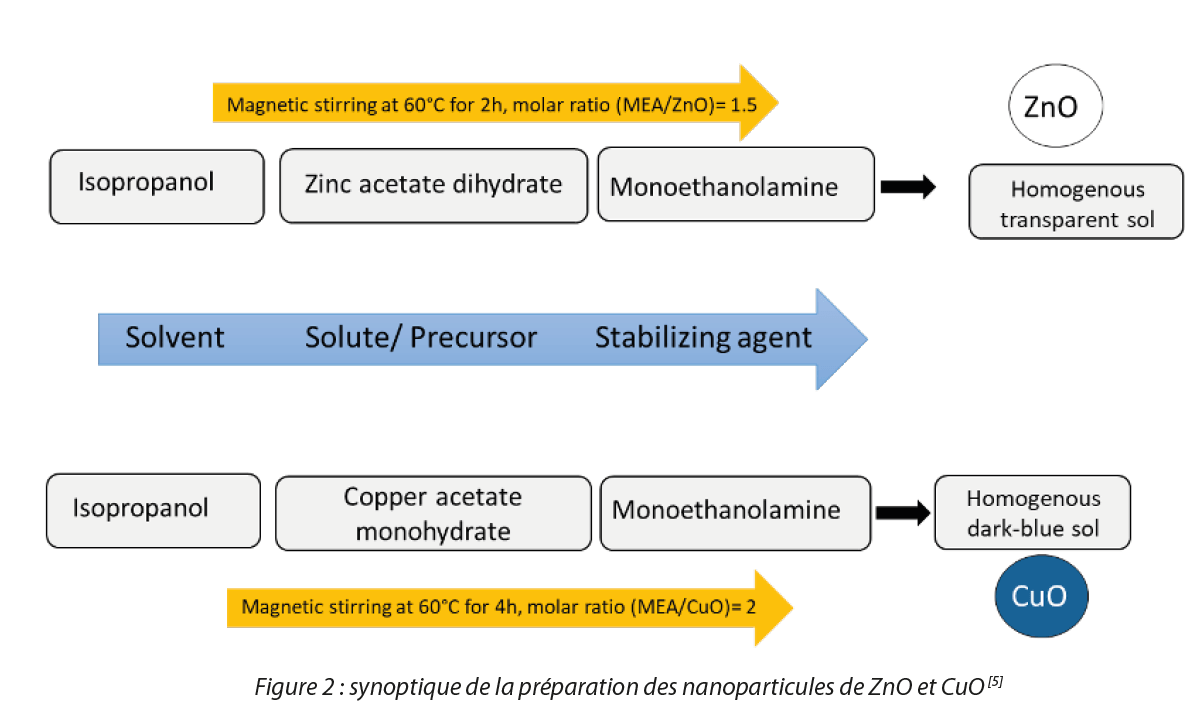
1. Synthesis of nanoparticles and thin films of CuO and ZnO
The sol-gel method was used for the synthesis of NPs of CuO and ZnO. CuO and ZnO solutions were prepared inside a glove box from zinc acetate dihydrate and copper acetate monohydrate dissolved in a mixture of isopropanol and monoethanolamine (MEA) used as a solvent and stabilizer respectively (Figure 2). The thin films were prepared by dip-coating on glass plates after prior surface preparation with sulfuric acid. The prepared thin films were heat treated at 500°C for one hour.
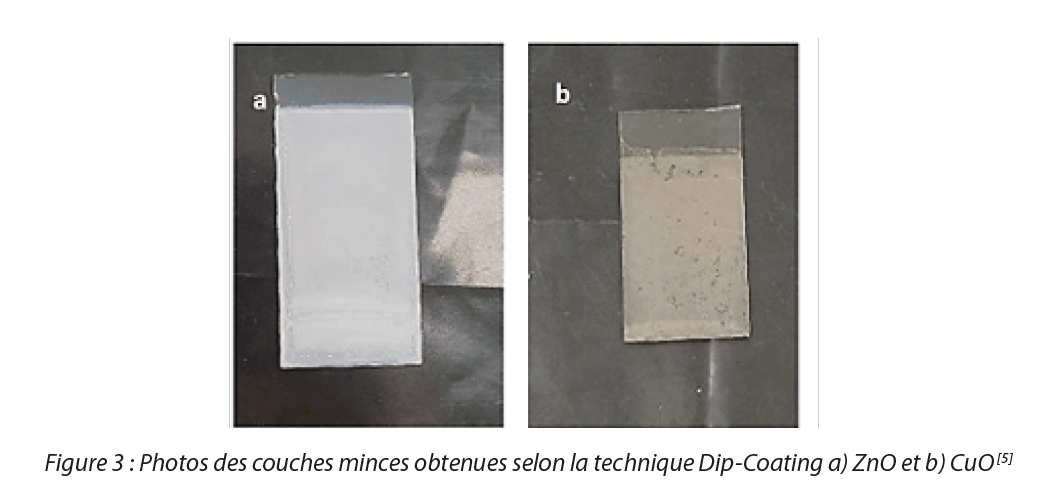
We obtained average sizes in the order of 3 nm for the two oxides in colloidal form. In the case of thin films, analysis by scanning electron microscopy (SEM) demonstrated that the deposits formed for CuO and ZnO are composed of particles with a diameter close to 30-40 nm, these films are homogeneous, dense and formed of spherical particles. The results of these characterizations confirmed the presence of a thin film of zinc oxide and copper oxide. Figure 3 shows the thin films obtained.
2. Tests of the antibacterial activity of NPs in colloidal solution and thin films
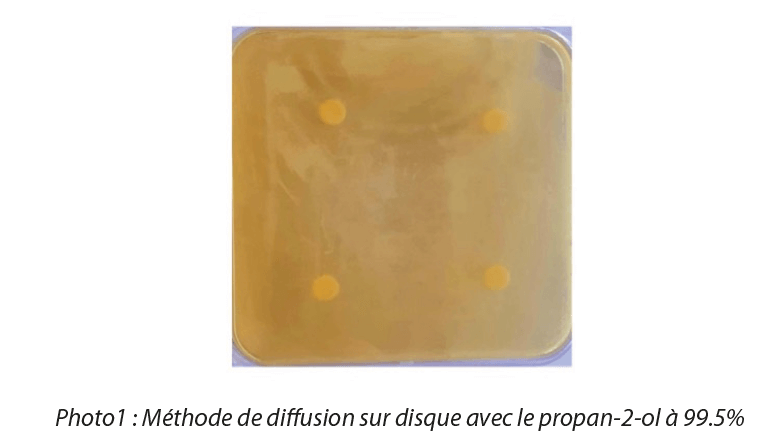
We used the disk diffusion method in the first instance to carry out testing of microbial susceptibility to nanoparticles. For these tests, we used NPs in suspension, and therefore in an amorphous form. The paper disks (6mm in diameter) were saturated with a colloidal solution of NPs at different concentrations with a volume of 20μL, then deposited on the surface of Petri dishes which were inoculated with different bacterial suspensions at a concentration of 107 CFU/mL, then incubated for 24 hr at 37°C to guarantee optimal growth conditions for the strains. The minimum concentration at which nanoparticles display antimicrobial activity is called the minimum inhibitory concentration (MIC) and the area around the disk where no bacterial growth is observed is called the zone of inhibition. A disk saturated solely with solvent was used as a control (Photo 1). Microbial susceptibility to metal oxide nanoparticles varied according to the microbial species and metal oxide concentrations.
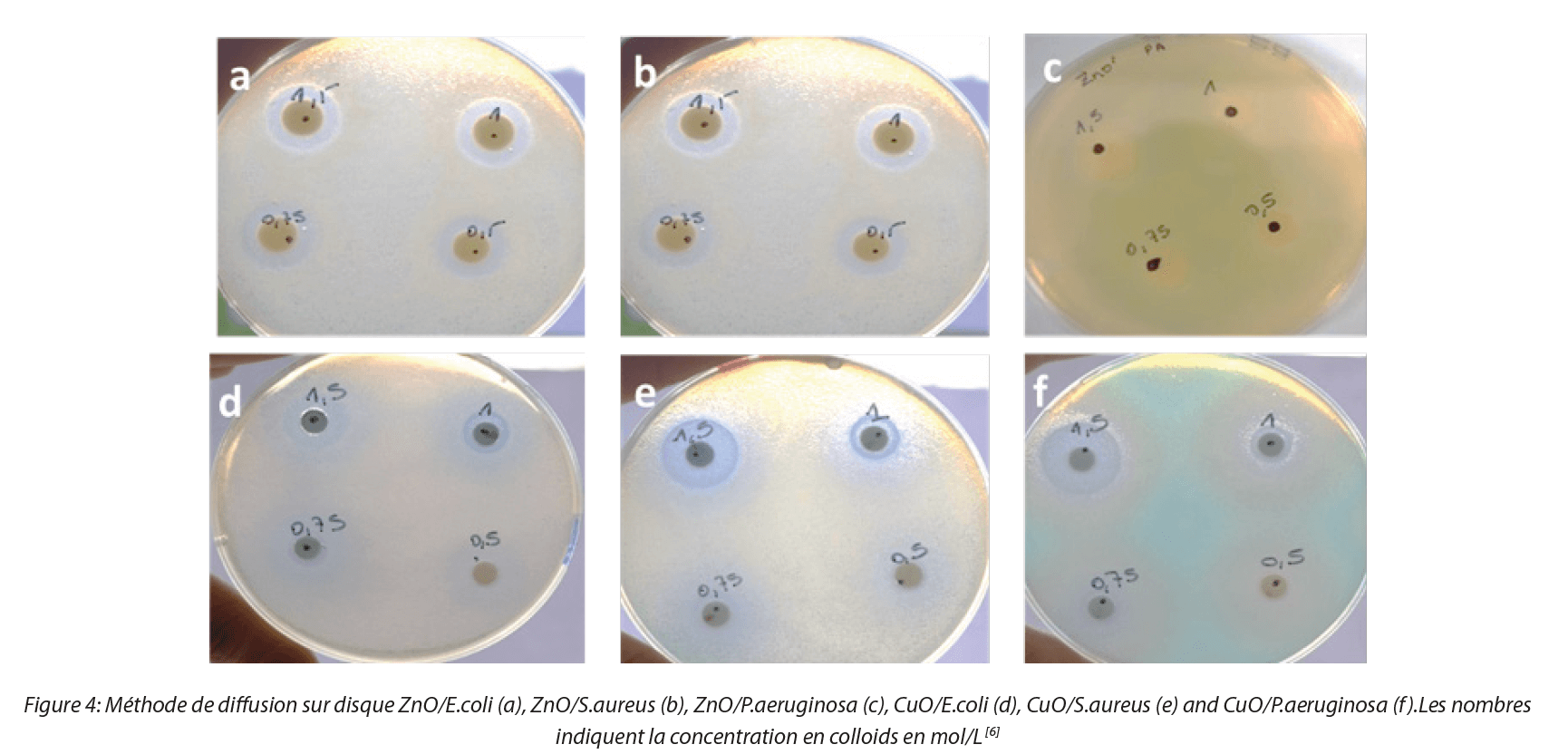
The results obtained are represented by Figure 4:
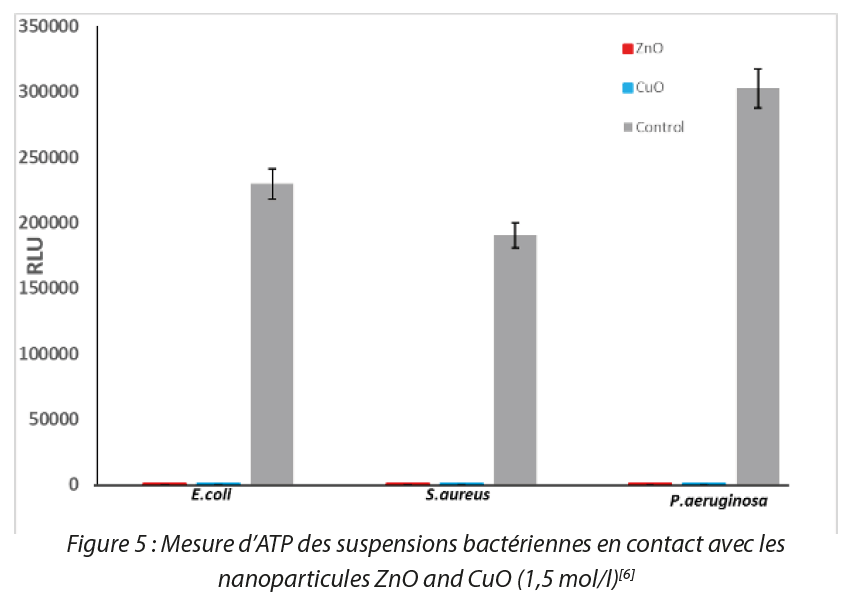
The results show that sols of CuO and ZnO nanoparticles behave as antibacterial agents with the presence of zones of inhibition. ZnO nanoparticles inhibit bacteria solely at sufficiently high concentrations, around 0.5 M while the antibacterial behavior of CuO nanoparticles begins at a lower concentration. These results were confirmed by the ATP-metry method, for which sol nanoparticle concentrations similar to those of the disk diffusion method were used. Measurements were made in 96 well plates after 50 μL of bacterial suspension at 109 CFU/mL and 50 μL of NP solution had been deposited in each well. Instantaneous measurements of ATP in the ZnO and CuO NP solutions converged towards zero for the three bacterial strains. The positive control (bacterial suspensions without NPs) was measured as a reference for each strain. Figure 5 shows a high value for ATP in the positive control with all strains in stationary phase.
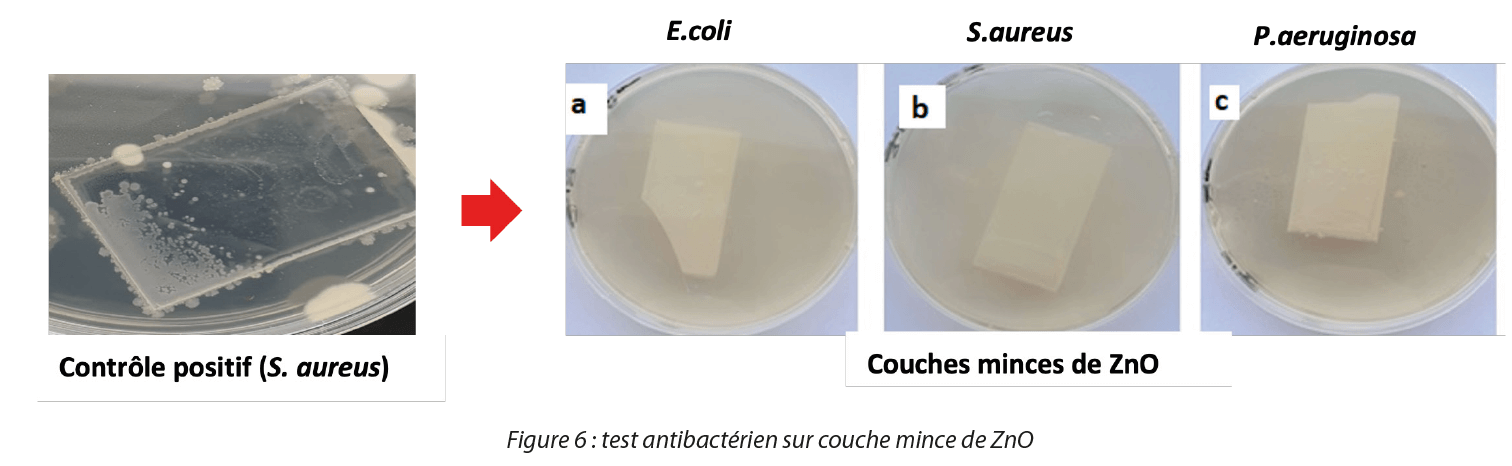
The RLU intensity of controls without bacteria is very high, and we observe a significant abatement on the red and blue histograms in Figure 5. Conversely a marked reduction in the ATP signal was observed with all samples in contact with ZnO and CuO NPs, which demonstrates their antibacterial action. For antibacterial tests on thin films, we developed a methodology that allows the evaluation of antibacterial activity in the form of thin films, taking inspiration from the European standard NF EN 14561 (2007-03)[7]. We validated our method with thin films of ZnO, CuO and TiO2 on the reference bacterial strains used in this work [8] as shown in Figure 6.
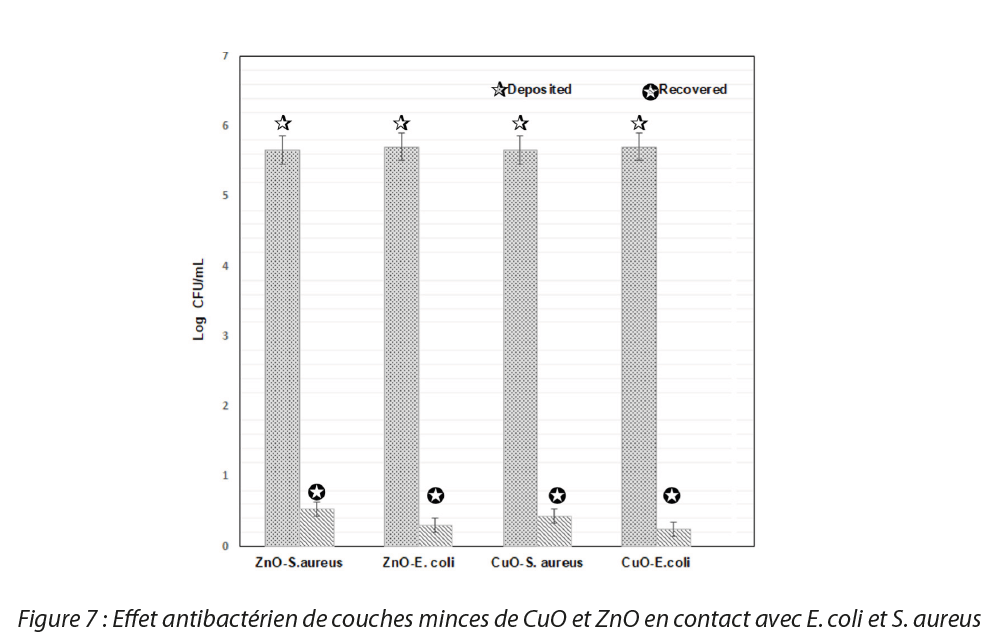
The results obtained after incubation of the slides are presented in Figure 7 and show a reduction in the viability of the strains studied when they are in contact with ZnO and CuO NPs. In fact, no bacterial growth was observed on the agars in which the slides bearing thin films of NPs were placed. The results show that the thin films of nanoparticles display high antibacterial activity. A 5-log reduction in the bacterial population deposited was noted for the 3 strains studied.
Conclusion
NPs of ZnO and CuO in solution display an antibacterial action against Gram-positive bacterial strains (Staphylococcus aureus) and Gram-negative bacterial strains (Escherichia coli and Pseudomonas aeruginosa). The results of the antibacterial tests on the thin films showed antibacterial efficacy for all the NPs against the different strains studied with a 5-log reduction in the bacterial population.
Several possibilities are envisaged for the continuation of this work:
- Expansion of these tests to fungal and viral species.
- Studies of the effect of nanoparticle size on antibacterial activity.
- Production of formulations with these nanoparticles leading to products with antibacterial properties (paints, packaging…).
- Performance and optimization of tests on prototypes in the form of thin films.
- Cross-referencing these results with the theme of biopolymers with the aim of developing antibacterial biomaterials.
References
[1] Izunna S. Okeke, Kenneth K. Agwu, Augustine A. Ubachukwu, Fabian I. Ezema, Influence of transition metal doping on physiochemical and antibacterial properties of ZnONanoparticles: A review, Applied Surface Science Advances,Volume 8,2022,100227
[2] L. Hall-Stoodley, J. W. Costerton, and P. Stoodley, « Bacterial biofilms: from the natural environment to infectious diseases », Nature Reviews Microbiology, vol. 2, no. 2, pp. 95-108, 2004.
[3] P. Watnick and R. Kolter, « Biofilm, city of microbes », Journal of Bacteriology, vol. 182, no. 10, pp. 2675-2679, 2000.
[4] S. Kheiri, X. Liu, M. Thompson, Nanoparticles at biointerfaces: Antibacterial activity and nanotoxicology, Colloids and Surfaces B:Biointerfaces, Volume 184,2019,110550, ISSN 0927-7765.
[5] Rania Dadi. Synthèse de nanoparticules d‘oxydes métalliques et leur activité antibactérienne. Matériaux. Université Paris-Nord – Paris XIII, 2019
[6] R. Dadi, R.Azouani, M.Traore, C. Mielcarek, A. Kanaev. Antibacterial activity of ZnO and CuO nanoparticles against gram-positive and gram-négative strains. Materials Science and Engineering: C,Volume 104,2019,109968,ISSN 0928-4931
[7] Norme NF 14651, Agence Française de Normalisation, Décembre 2007
[8] C. Mielcarek, R. Dadi, A. Roynette, A. Lemarchand, A.Kanaev, K. Senni, M.Traore, R. Azouani. Antibacterial Activity Evaluation of ZnO, CuO, and TiO2 Nanoparticles in Solution and Thin Films Bioluminescence, 2022, Volume 2525.ISBN : 978-1-0716-2472-2
Glossary
ATP: adenosine triphosphate
CMI: minimum inhibitory concentration
CuO: copper oxide nanoparticles
ERO: reactive oxygen species
MEA: monoethanolamine
NPs: nanoparticles
RLU: relative light unit
TiO2: titanium oxide nanoparticles
UFC: colony forming unit
ZnO: zinc oxide nanoparticles
Share

Imroi EL-HABIB, Anne ROYNETTE, Christine MIELCAREK, Alex LEMARCHAND & Mamadou TRAORE &
Rabah AZOUANI