October 2024
La Vague n°83
EU GMP Annex 1 / Patient risk management ICH Q9(R1) / Digitization
Summary
- What does 21 CFR Part 11 mean in everyday online analytics?
- Digitalization in cleaning validation an overview of possibilities, challenges, and opportunities for savings?
- EU GMP Annex 1. Implementation of Contamination Control Strategy
- Impact of the new Annex 1 on Sterile Filling
- The Challenges of Floor Cleaning & Sanitization
- Réduction énergétique des centrales de traitement d’air : comment adapter son monitoring environnemental ?
- USP <922> Water Activity: A Better Approach for Lyo Moisture Determination. Applications enabled by rapid non-destructive headspace moisture analysis of freeze-dried product
- Sustainable water management in the pharmaceutical industry
- Rapid Testing for Cell & Gene Therapy Products: A Three-Level Approach Using an Automated Solid Phase Cytometry System
Digitalization in cleaning validation an overview of possibilities, challenges, and opportunities for savings?
Within the pharmaceutical industry, there has been a significant surge in articles and publications related to digitalization, with abundant references to concepts such as Pharma 4.0. This article intends to shine a spotlight on the ongoing discussions surrounding digitalization within the realms of cleaning validation, overall hygiene protocols, and adjacent quality assurance activities.
Our objective is to map out the potential benefits and underscore the advantages that emerge when transitioning from traditional paper-based systems or the use of non-validated digital tools and manually managed documents to an environment where these processes are supported by validated digital solutions.
Through this article, we aim to raise awareness about the prevalence of manual and paper- based protocols within the industry. Very often, there is a lack of dialogue among relevant stakeholders and cross-functional teams regarding the digitalization of these processes. This may be attributed to the entrenched belief that these methods, having been established in the organization for years, are both efficient and effective as they stand.
1. Digitalization in Cleaning Validation: Current Worst-Case Identification
Cleaning validation is a pivotal aspect of quality assurance in pharmaceutical manufacturing. It plays a crucial role in ensuring that manufacturing equipment is properly cleaned to prevent cross-contamination between products, which can have possible implications for patient safety, product integrity, and its effectiveness.
To initiate a cleaning validation project in pharmaceutical manufacturing, a set of fundamental activities must be undertaken. These tasks often involve intricate calculations, tables, and data analysis, all hinging on defined perspectives of logical interrelationships. For example, among these activities are:
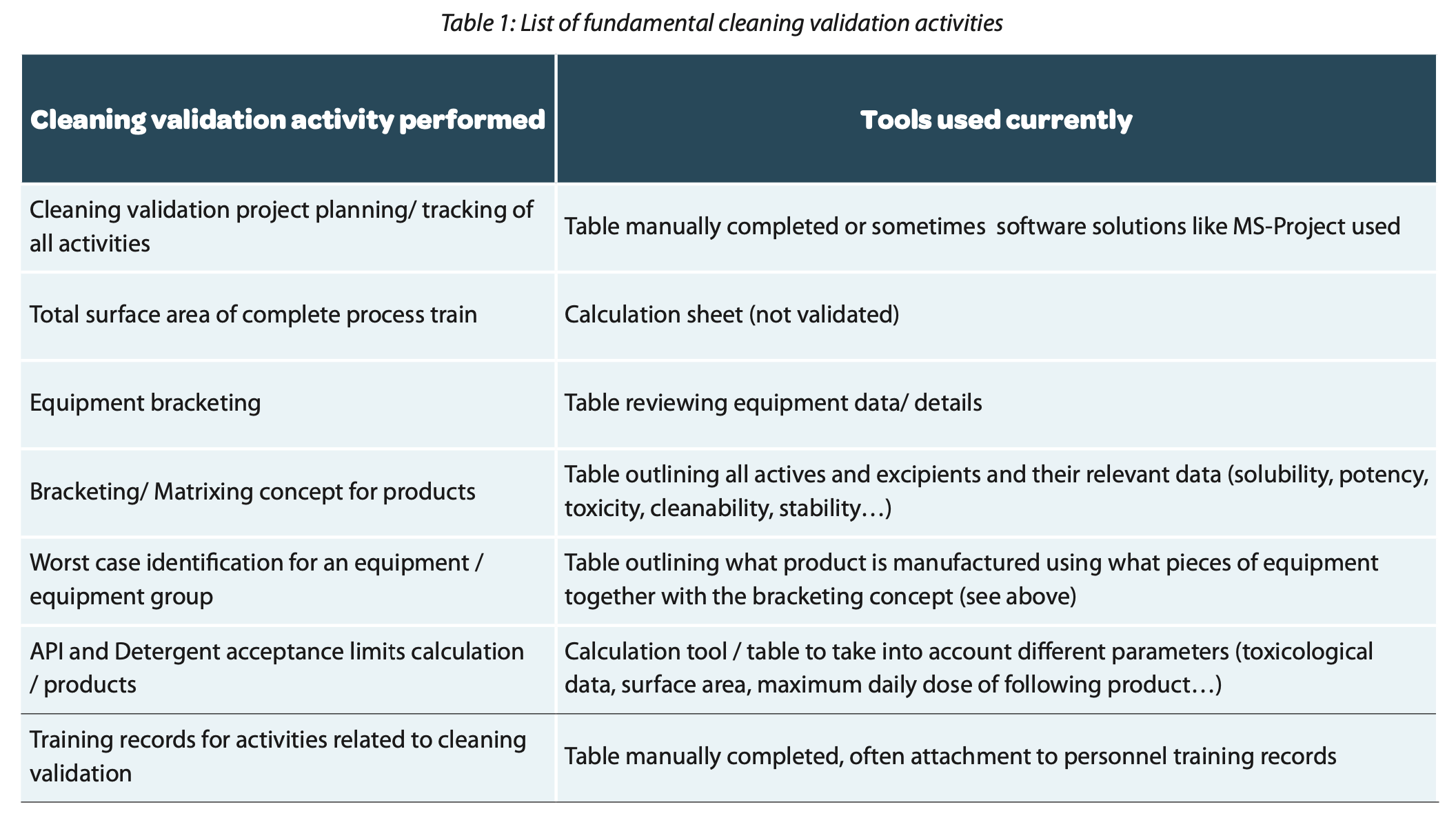
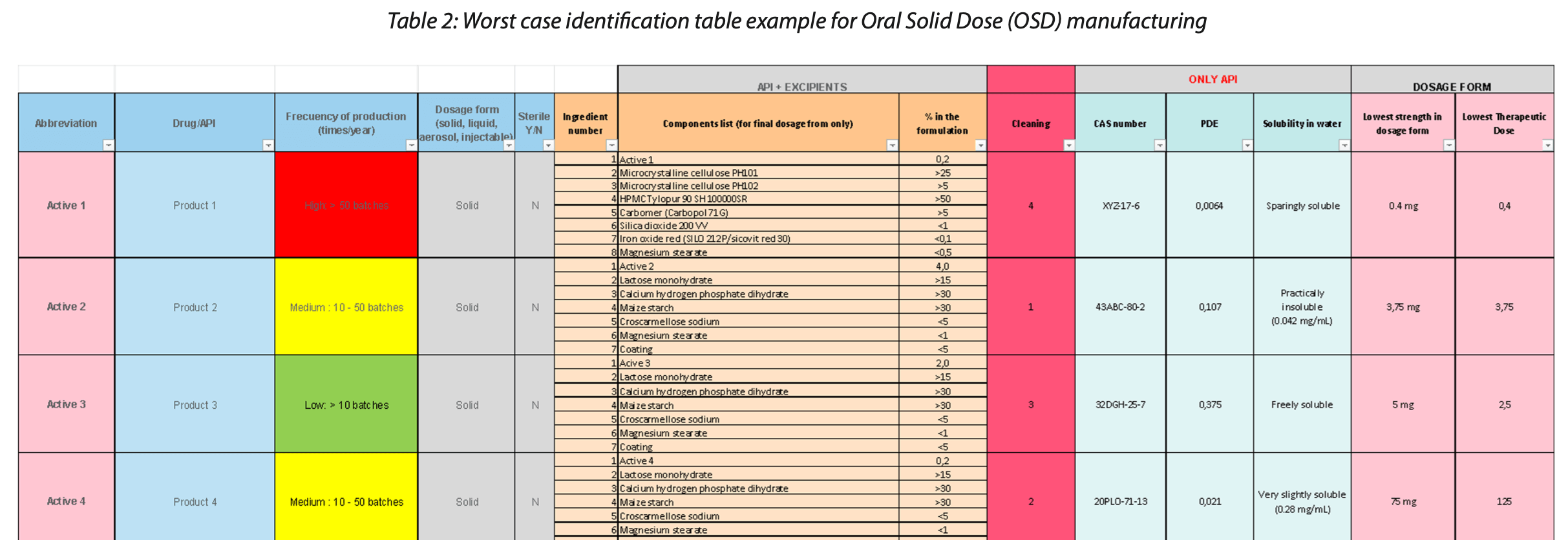
For these activities validated digital tools can be a significant improvement on data integrity. We want to explain this statement with an example of a table used for worst case identification (refer to Table 2).
The table above is an example of how the worst-case product identification assessment in OSD manufacturing is currently performed. The relevant parameters outlined in the guidelines (i.e., in EU-GMP Annex 15 section 10.10[1]) are considered. Information on the PDE for each of the active ingredients, cleanability and solubility data can be found in Table 2.
Questions related to these types of tables might be:
1. Is the source of the data known?
2. Who entered the data?
3. Is the data approved by appropriate subject matter experts? 4. When and who did the last change of the data?
5. Is there a procedure to initiate workflows when the figures or calculations need to be updated in the table?
6. How is the company making sure that all relevant colleagues are working with the latest version of the table?
To address these challenges effectively, various approaches have been observed. In the context of paper-based systems, the most time-consuming task is typically the document approval workflow. This process involves printing the document and circulating it among relevant stakeholders for their signatures—often a cumbersome and slow procedure.
Subsequent steps in the document management process include the storage of the physical hard copy in an archive room, as well as a scanned version in a digital location, such as a SharePoint site. These measures, however, present multiple difficulties, particularly when the content of a document needs to be updated—for example, when introducing a new product. The author is then required to verify that they are working with the correct version of the document by comparing the archived hard copy, the scanned copy, and the most current electronic file.
To overcome these highly inefficient and error-prone practices, companies may turn to alternatives such as password-protected files or electronic document management systems (DMS). Through such systems, the validated and approved documents are stored and made accessible in a more streamlined and secure manner.
2. Digitalization in cleaning validation – worst case identification
Implementing a validated digital tool can address several concerns associated with traditional paper-based documentation processes. Here are a few ways in which a digital system can improve efficiency and compliance:
1. Document Generation: The system enables the creation of specific user profiles with varying levels of access, such as author, reviewer, and approver. This ensures that only designated individuals identified by the organization can access documents and perform relevant tasks, thereby maintaining control and integrity throughout the document lifecycle.
2. Change History: With each user logging into the system using their unique credentials, all actions taken on a particular document can be tracked immediately. This feature captures who made changes, when they were made, and the reasons behind those changes, ensuring full traceability and greatly improving Good Documentation Practice (GDP) compliance.
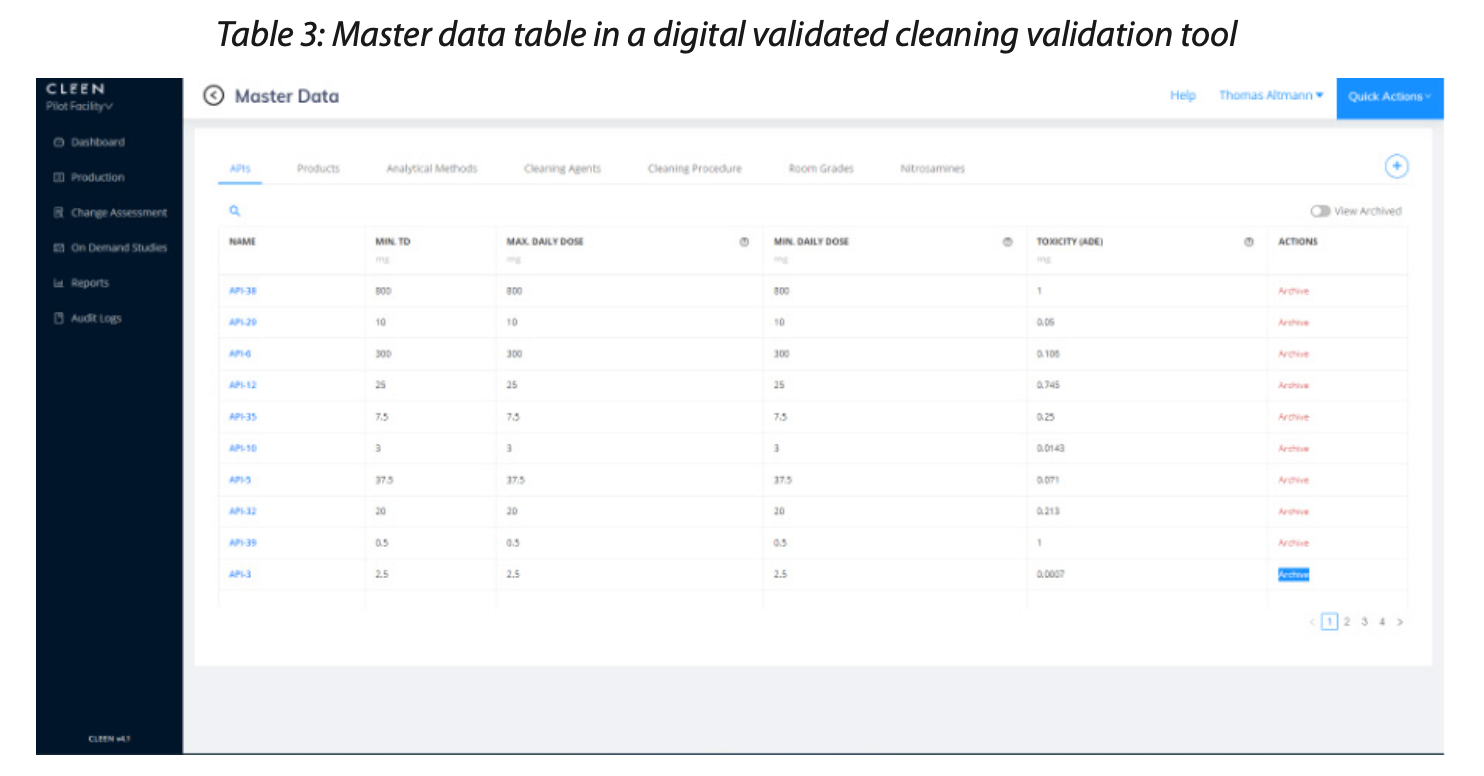
For example, when identifying the worst case in cleaning validation, the initial step in a validated digital software solution would be to enter the so-called master data into the system. This creates an accurate and immutable record right from the outset, streamlining the subsequent steps and ensuring consistency across the entire process.
The table above provides a list of master data necessary for conducting a worst-case assessment. The scope and detail of the master data hinge on the strategy employed for determining the worst case, which bears similarities to the manual tabulation method previously discussed. However, the use of master data within validated software offers distinct advantages.
One key benefit of validated software is that users are prevented from arbitrarily adding or deleting parameters simply because they seem pertinent to a specific report. This level of control means that a minimum data set — the master data — must be input and authorized by designated individuals. This requirement ensures consistency, as the worst-case identification is invariably executed using the same data set across all products and equipment.
In our interactions with quality assurance professionals, we’ve learned that they appreciate having definitive guidance regarding the required master data for worst-case identification. In the current landscape, there is considerable uncertainty about what elements must be evaluated, often leading stakeholders to consider excessive and potentially irrelevant data. This additional scrutiny can result in prolonged times to complete the assessment using the traditional manual methods. Conversely, in a digital software environment, there is clear instruction on which parameters need to be assessed, which can expedite the completion of each assessment.
Utilizing the master data along with the software’s built-in logic for isolating the worst-case product, the digital system consistently identifies the most critical case using the same criteria. Consequently, the software can pinpoint the worst-case product for each piece of equipment, and this determination can be conveniently displayed within the software interface.
The use of digital tools for cleaning validation activities, such as worst- case identification, shows the potential benefits of transitioning from a paper-based system to a digitalized approach.
One of the primary advantages is increased compliance, facilitated by having a real-time history log where all actions can be easily traced. This ensures that data are always Attributable, Legible, Contemporaneous, Original, and Accurate (ALCOA).
Digital tools provide a consistent methodology for identifying the worst-case product, tailored to the user’s requirements. This uniformity is not just limited to worst-case identification but also extends to other critical cleaning validation activities. These include the calculation of limits and the establishment of maximum safe carryover limits, as well as the determination of the total surface area of the entire process train.
By leveraging these digital advantages, organizations can achieve a higher standard of data integrity and process efficiency, ultimately enhancing the quality assurance of their cleaning validation procedures.
3. Digitalization of hygiene activities
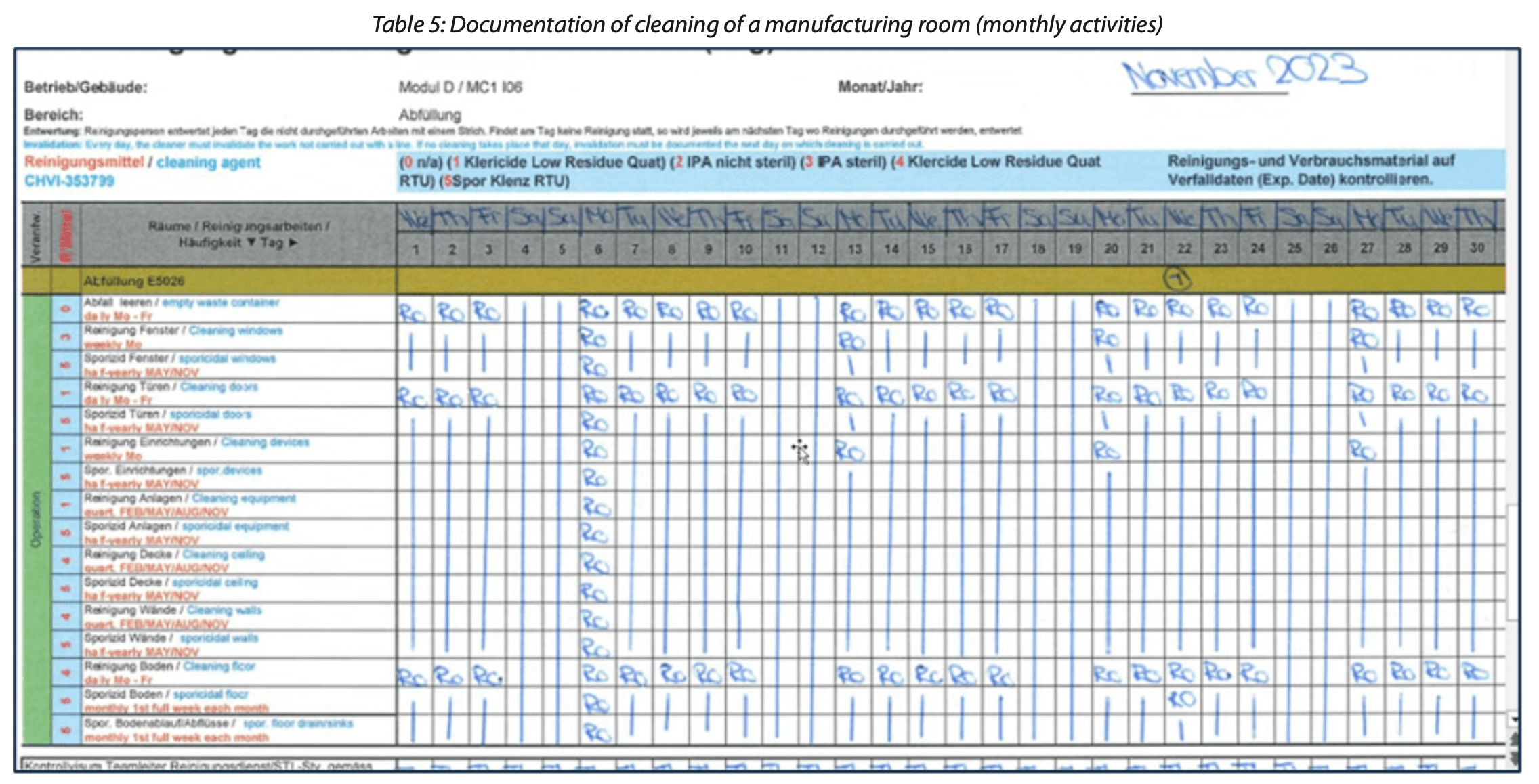
In addition to documentation associated with cleaning product contact surfaces and cleaning validation activities, there are other hygiene-related actions within manufacturing environments that necessitate thorough documentation. One such example is the cleaning of manufacturing rooms. The current practise to document the location/room where cleaning shall be performed is to use forms/ logbooks like the one shown below in Table 5.
The example above illustrates how cleaning activities, which are listed on the left side of the table, are recorded by the initials and signatures of the operator who carried them out. However, this approach fails to capture essential details such as the duration of the cleaning task or the exact time it was performed, as evidenced by their absence from Table 5.
Being a paper-based form, it is only accessible in the specific manufacturing room where the cleaning takes place. Consequently, reviewing this form requires physical presence in the storage area. It is not uncommon for these forms to go unchecked on a daily basis, which can lead to delayed identification of non-compliance issues, such as inadvertently skipped cleanings.
When non-compliance is eventually detected, it triggers an investigation. However, significant delays between the occurrence of the event and its discovery can hinder the investigative process, as personnel may struggle to recall the specifics of the incident, including what happened, when it happened, and why.
La Vague 83.indd 19
Implementing a digital software solution for manufacturing room cleaning activities can greatly enhance the monitoring of the rooms’ current status, indicating whether they are clean, dirty, or in the process of being cleaned. It also facilitates the tracking of the time required to complete a cleaning task. By utilizing a digital tool, there is immediate clarity on all executed activities, greatly improving oversight and the timely identification of any issues.
4. Digitalization of other activities
Beyond the above, it’s important to recognize that a variety of quality-related activities are systematically recorded using logbooks or standardized forms. To illustrate this, let’s consider monitoring the environment in a storage room for raw materials. In our scenario, operators are required to record the temperature and humidity levels of the storage room once per shift.
On the form sheet above, you will observe fields that operators must complete by hand. In addition to recording the temperature and humidity, the operator is also required to note down the time of sensor inspection, alongside their initials and the date. The fields for temperature and humidity are highlighted in orange because these are the critical pieces of information needed. The fields highlighted in blue, on the other hand, are there to confirm when and who performed this reporting task.
Should this process be conducted using validated digital software, the operator would first log in with unique credentials. Upon accessing their daily schedule, the task for measuring humidity and temperature would be listed. The operator would then read the sensor measurements and input the values directly into the system, which would automatically capture and store details about who performed the task and when.
Utilizing such a digital tool not only streamlines data capture but can also facilitate the statistical evaluation of the data over time. While the paper form sheet is typically filed and archived unless out-of- specification data are detected, digital tools offer enhanced capabilities. They provide a clear visualization of temperature and humidity trends, potentially across different seasons, offering insights into the consistency of these values. This may even allow for adjustments in the frequency of checks, potentially reducing the workload for operators.
5. Digitalization of cleaning validation – What are the opportunities of savings?
Moving away from paper-based processes, such as cleaning validation and logbook activities, to a digital software tool can significantly streamline operations. The following time-consuming activities may be eliminated with the adoption of digital solutions:
1. Preparing the document, including updates to dates and other relevant details.
2. Printing the document; this also has a sustainability benefit, as it reduces paper use.
3. Manually amending the printed document, if necessary, which may require additional handwriting.
4. Physically distributing the printed document to the location where it is needed.
5. Manually completing the paper forms, logbooks, or instructions.
6. Collecting the completed documents for later review and archiving purposes.
7. Having the documents reviewed by the person responsible for quality control.
8. Archiving the physical document, which includes filing and managing storage space.
9. Locating the document during inspections or audits, a process that can be time-consuming and challenging if records are extensive.
Remarkably, we have found that a pharmaceutical manufacturing site with approximately 500 employees prints over 170,000 sheets of paper every year. With all the handling steps mentioned above, this equates to roughly three Full-Time Equivalents (FTE) dedicated solely to managing these printouts.
Conclusion
This article has explored the potential of using a validated digital software tool, to manage a wide array of activities, from cleaning validation, like worst-case scenario identification and limit setting, to environmental hygiene monitoring and even routine logbook tasks. The most significant advantage of utilizing a digital tool is the enhanced quality control it offers by having all data readily accessible at any time. Implementing validated digital software enables the evaluation of data through statistical tools or other methods, ensuring that all site activities are under control, thus safeguarding both patient health and the quality of pharmaceutical products.
Share