October 2024
La Vague n°83
EU GMP Annex 1 / Patient risk management ICH Q9(R1) / Digitization
Summary
- What does 21 CFR Part 11 mean in everyday online analytics?
- Digitalization in cleaning validation an overview of possibilities, challenges, and opportunities for savings?
- EU GMP Annex 1. Implementation of Contamination Control Strategy
- Impact of the new Annex 1 on Sterile Filling
- The Challenges of Floor Cleaning & Sanitization
- Réduction énergétique des centrales de traitement d’air : comment adapter son monitoring environnemental ?
- USP <922> Water Activity: A Better Approach for Lyo Moisture Determination. Applications enabled by rapid non-destructive headspace moisture analysis of freeze-dried product
- Sustainable water management in the pharmaceutical industry
- Rapid Testing for Cell & Gene Therapy Products: A Three-Level Approach Using an Automated Solid Phase Cytometry System
Rapid Testing for Cell & Gene Therapy Products: A Three-Level Approach Using an Automated Solid Phase Cytometry System
Cell and gene therapies represent cutting- edge treatments in regenerative medicine, providing innovative solutions for conditions previously considered untreatable. Due to the live nature of these products, terminal sterilization is not feasible, making sterility testing a critical component to ensure the safety of CGT products prior to patient administration. Additionally, these therapies possess a notably shorter shelf life compared to traditional pharmaceuticals.
The conventional compendial sterility test, which requires 14 days for results as outlined in the European Pharmacopoeia (Eur. Ph.) 2.6.1[1], is inadequate for such time-sensitive applications. This underscores the urgent need for the adoption of rapid microbiological methods capable of delivering timely results, as highlighted in (Eur. Ph.) 2.6.27[2].
To address this need, the Red OneTM solid-phase cytometer has been developed, offering a rapid and efficient solution by detecting microorganisms through metabolic activity-based staining. A major challenge in this context is due to the presence of esterase enzymes in both mammalian cells, which are intrinsic to CGT products, and microorganisms. Redberry’s innovative approach addresses this challenge by employing a reagent that selectively lyses mammalian cells without compromising microbial integrity, thereby enabling accurate and rapid differentiation between host cells and contaminants.
Several workflows have been developed, allowing three levels of contamination risk management with time-to-result (TTR) ranging from 10 minutes to 4 days. Collaborative evaluations with CGT manufacturers across various cell types, including HeLa, Jurkat, Stem, CAR-T, and CHO cells, have demonstrated the efficacy of these workflows in detecting microorganisms in alignment with pharmacopeia guidelines [1].
1. Red OneTM Technology: The New generation of Solid Phase Cytometry
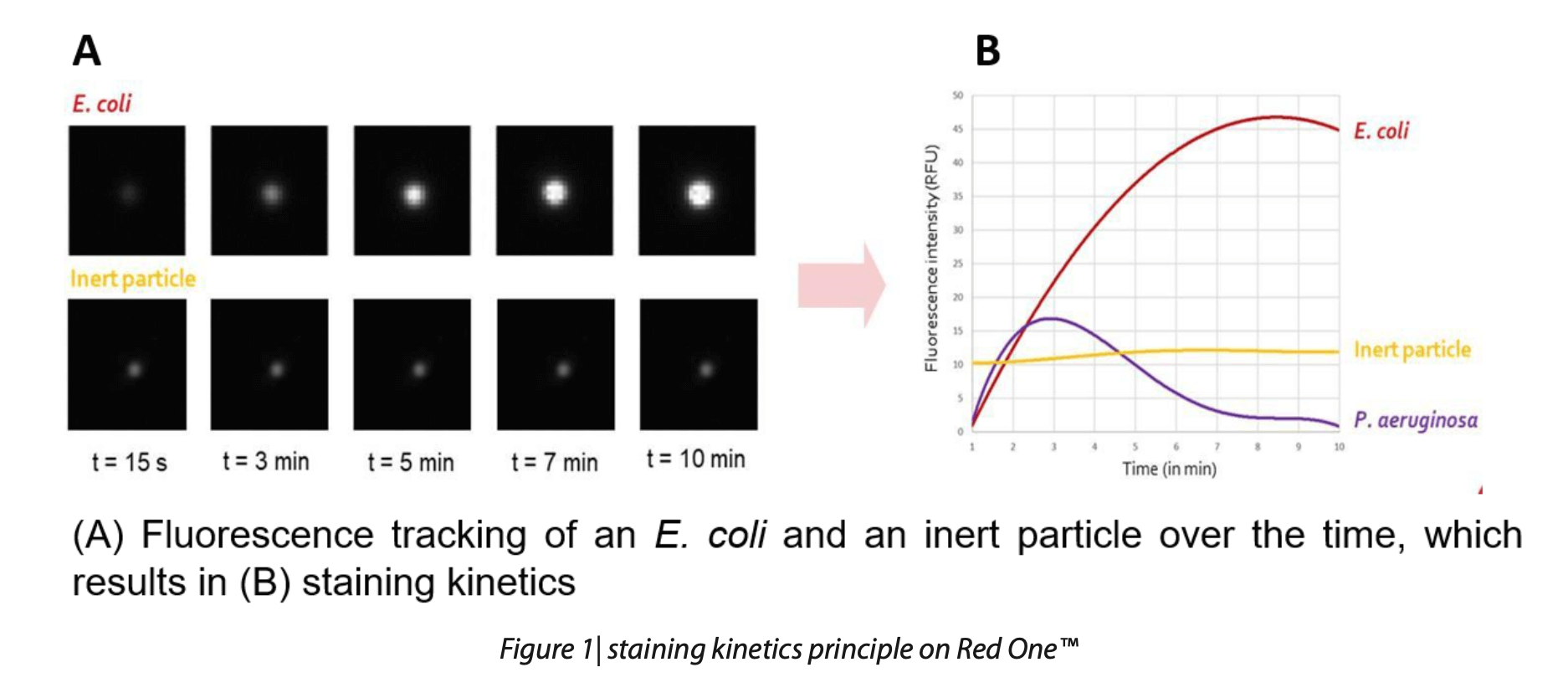
The Red OneTM platform is a fully automated microbiology system that detects single stained cells based on their metabolic activity. Designed for compatibility with standard laboratory setups, the Red OneTM system easily integrates into laminar flow hoods and has demonstrated high reliability in detecting individual cells by monitoring their staining kinetics. The system employs advanced image processing technology to distinguish viable cells from inert debris with high precision.
Utilizing a fluorescein derivative sensitive to esterase activity, combined with a high-resolution CMOS camera and powerful LED illumination, the Red OneTM system captures high-resolution images of microorganisms before, during, and after the application of the viability staining agent, within a 10 to 15-minute timeframe. This patented staining kinetics monitoring ensures reliable differentiation between viable cells and non-viable particles, such as those exhibiting auto-fluorescence (Figure 1).
The system is capable of processing sample volumes ranging from 10μL to 250 mL using single-use caps with track-etched PET membranes (porosity: 0.4 μm). All operations, including filtration and staining, are fully automated, eliminating the need for calibration.
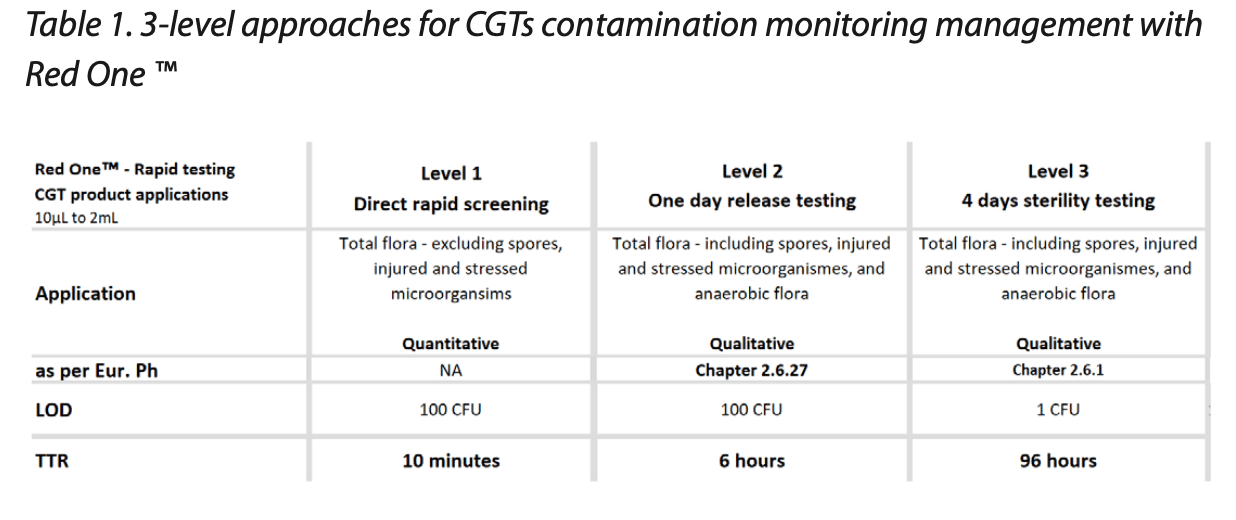
Three primary approaches were developed and evaluated for rapid microbiological control of CGTs (Table 1).
These 3-level approaches provide different levels of information for effective contamination risk management, as discussed below.
2. CGT Matrices Pre-Treatment: Reducing Mammalian Cell Background Noise
Given the inability to differentiate between microorganisms and mammalian cells using fluorescein derivatives alone, a pre-treatment step for CGT matrices is necessary. The developed lysis reagent selectively targets and lyses mammalian cells, thereby releasing esterase from their cytoplasm and eliminating potential interference during SPC analysis, while preserving the viability and metabolic activity of microbial cells.
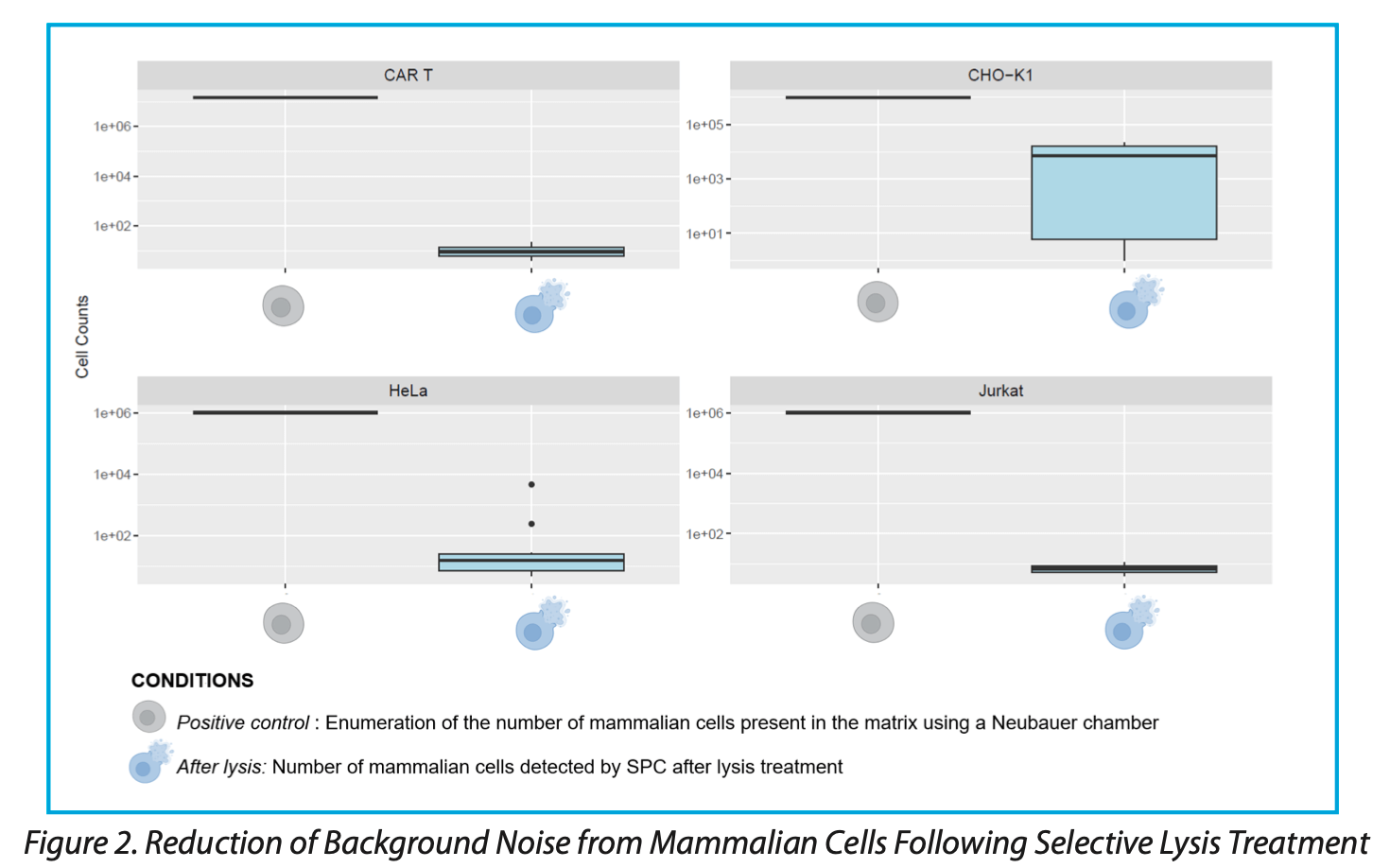
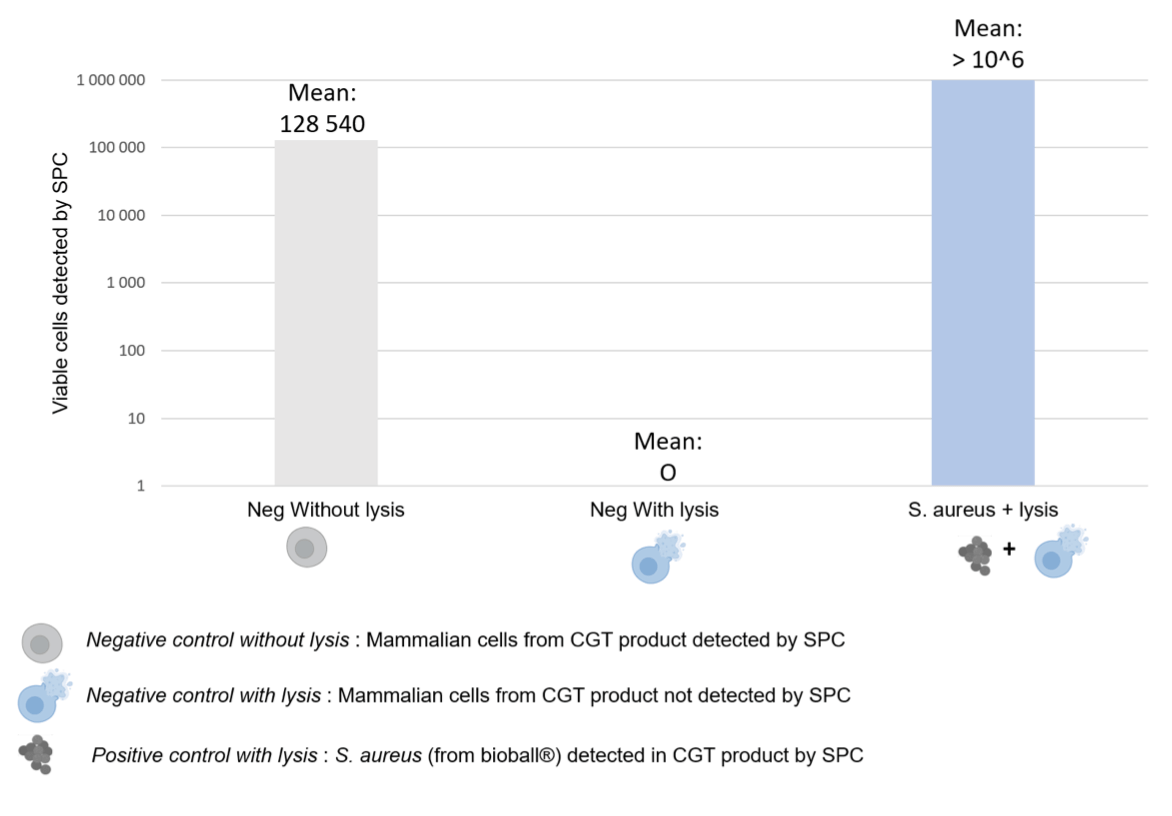
Application of the lysis reagent results in a significant reduction in the number of mammalian cells detected by SPC, exceeding 99.99% reduction compared to untreated controls (Figure 2). Given the system’s detection limit, concentrations above 5 × 105 to 106 cells/mL require initial calibration using a microscope and Neubauer counting chambers for accurate cell quantification.
The efficacy of this approach varies with cell type and concentration. For instance, the lysis reagent is particularly effective against CAR-T and Jurkat cells, both T lymphocytes, whereas CHO-K1 cells, with their more robust cellular architecture, demonstrate greater resistance to lysis (possibly due to their more robust cellular architecture, including thicker membranes and stronger cytoskeletal structures, which confer greater resilience against the lysis process).
Overall, the developed lysis reagent significantly reduces the number of detected mammalian cells post-lysis, thereby facilitating the detection of low levels of microbial contamination in CGT products. For some CGT products, it may be necessary to adapt the lysis treatment. Incorporating a mechanical separation step prior to lysis can significantly reduce background noise from non-target cells, thereby enhancing detection accuracy. This mechanical separation can be achieved using filters with larger pore sizes, which effectively remove larger cells before the lysis treatment is applied.
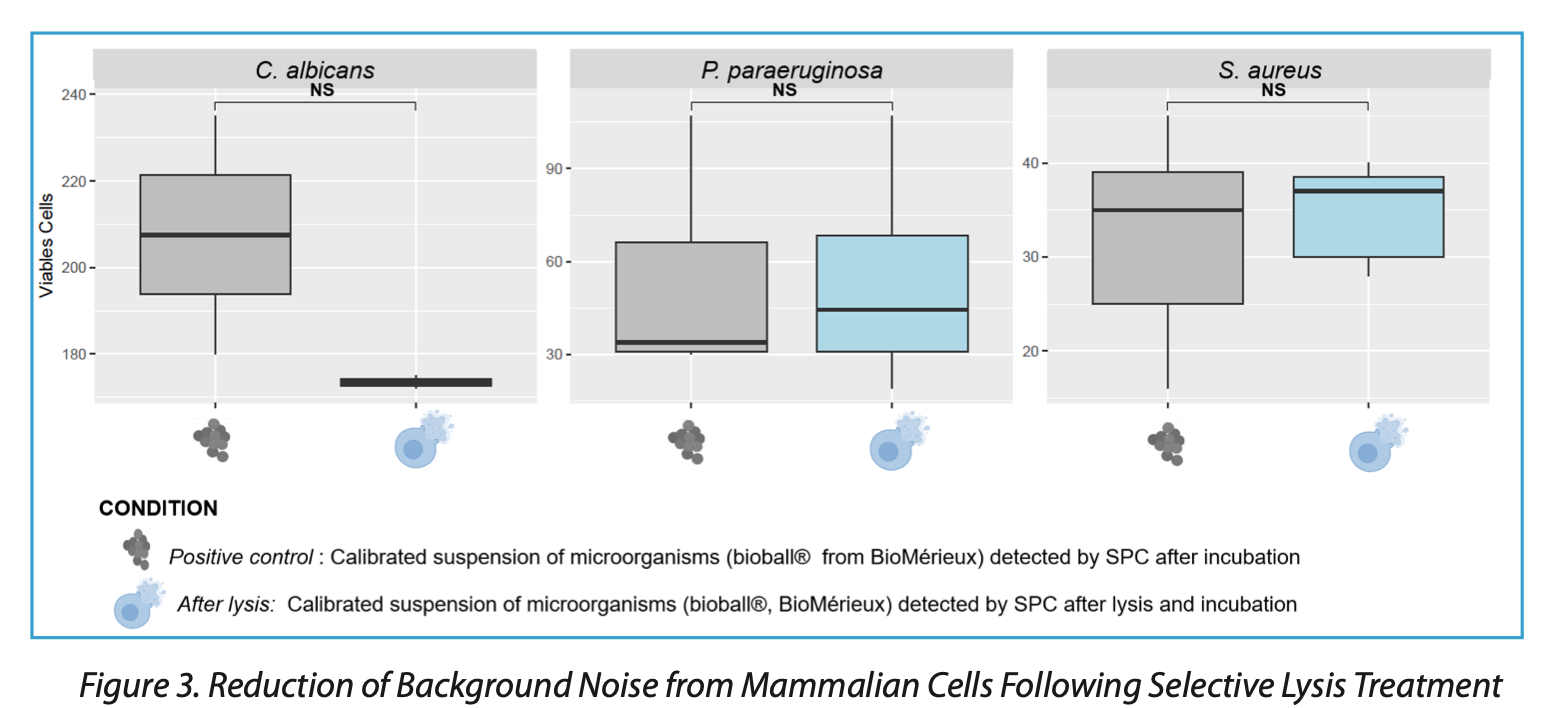
As illustrated in Figure 3, following an incubation period, no significant difference is observed in the SPC detection of Pseudomonas paraeruginosa (formerly P. aeruginosa), Staphylococcus aureus, and Candida albicans before and after lysis. Thus, the lysis reagent does not appear to adversely affect the detection of these microorganisms while effectively reducing the presence of mammalian cell contamination.
3. Contamination Risk Monitoring Strategies
Level 1: Direct Rapid Screening
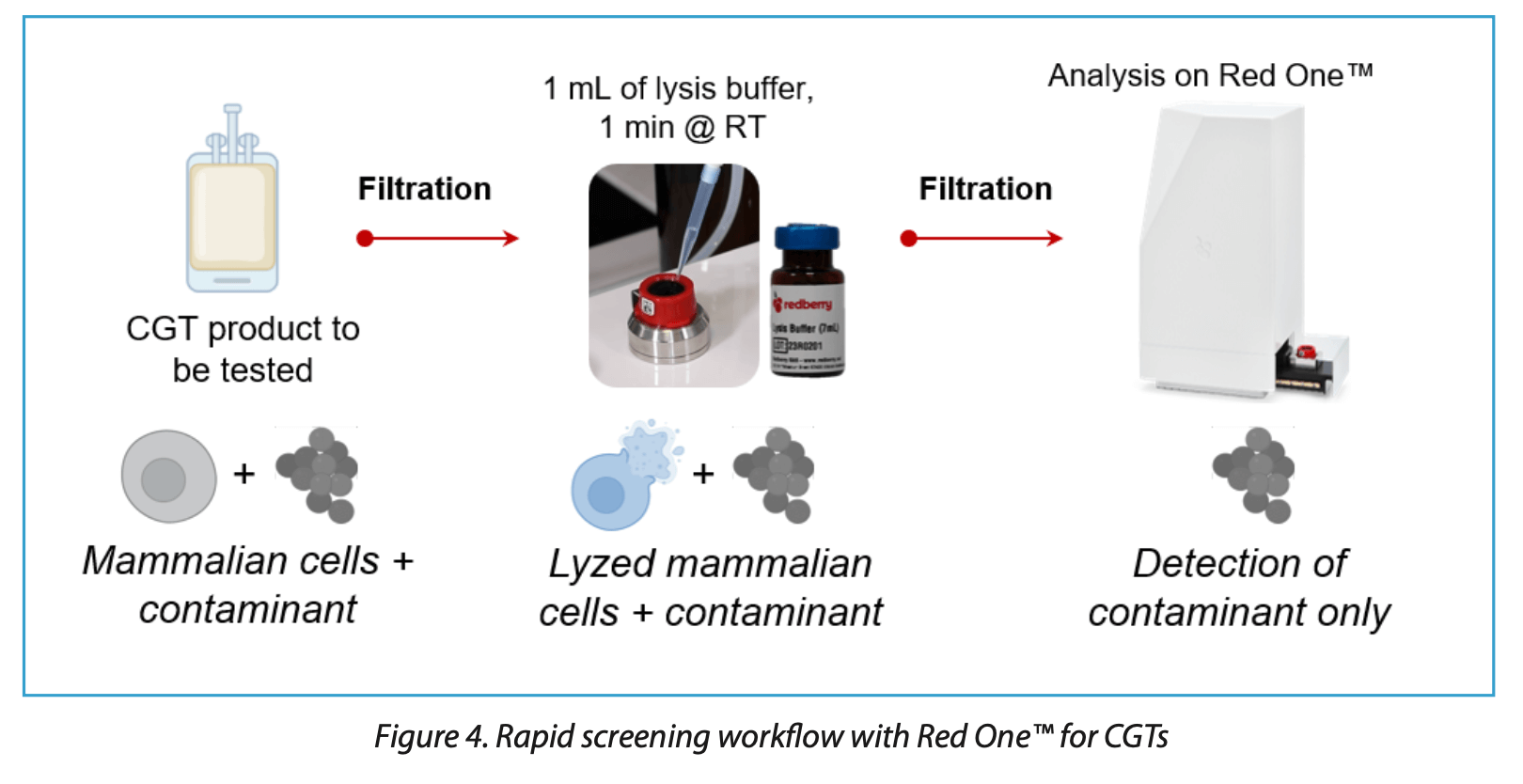
This approach allows the rapid screening of cell cultures for contaminants, providing direct counts of non-spore forming and non- stressed microorganisms within 10 minutes for sample volumes of 10 μL to 2 mL containing up to 107 cells (Figure 4). This method achieves over 99.99% reduction in mammalian cell detection, allowing effective differentiation between host cells and microbial contaminants. A limitation of this approach is its reduced sensitivity to spores and stressed or injured microbial cells, which may not exhibit sufficient esterase activity for reliable detection.
This approach ensures rapid detection of potential contaminations, facilitating prompt decision-making in critical situations.
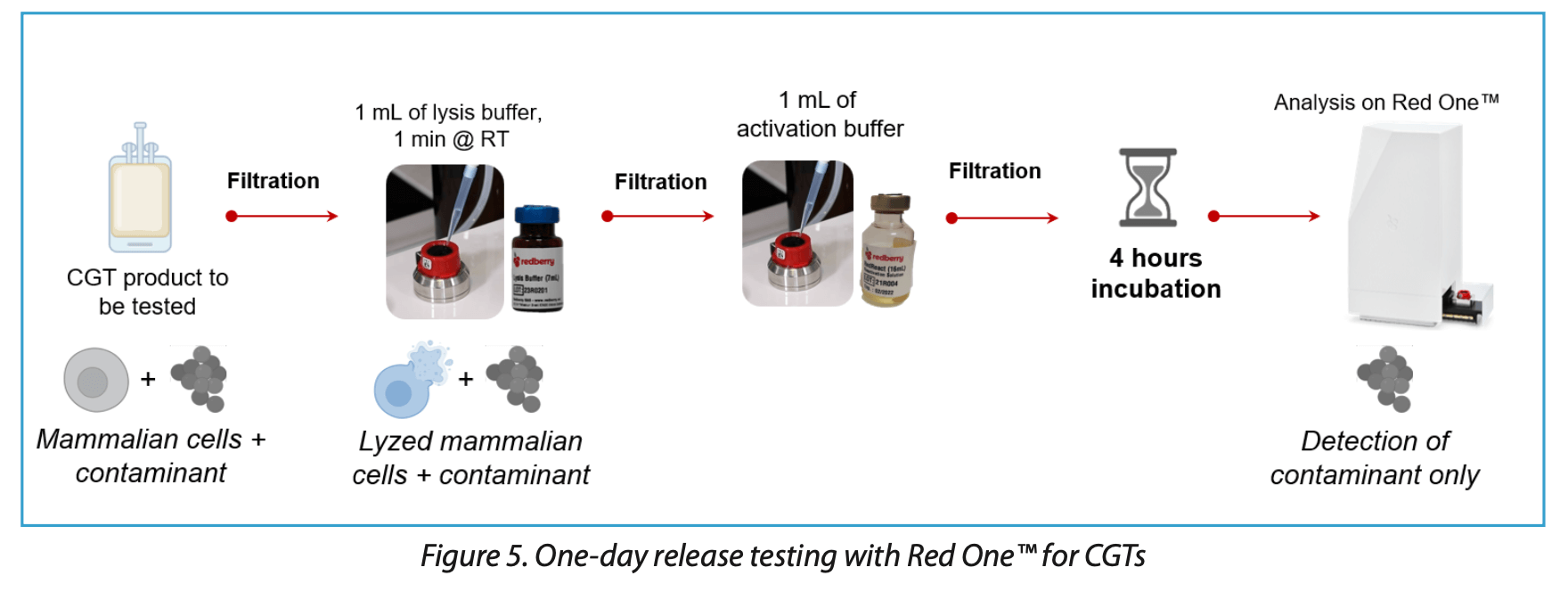
Level 2: One-Day Release Testing
This workflow, compliant with the detection limits outlined in Eur. Ph. 2.6.27, includes an enrichment phase for both aerobic and anaerobic microorganisms, enabling detection within less than 6 hours at a sensitivity of 100 CFU. This approach offers a significantly faster alternative to traditional methods; however, it does pose challenges in detecting certain anaerobic strains, such as Clostridium sporogenes and Cutibacterium acnes.
To effectively detect stressed microbial flora and spores, a specific protocol involving an incubation phase has been developed (Figure 5), aligned with the requirements of Chapter 2.6.27 of the European Pharmacopoeia. For the detection of aerobic microorganisms, including yeasts and molds, a 4-hour incubation is essential to ensure spore germination and the activation of sufficient metabolic activity. In contrast, detecting anaerobic microorganisms necessitates a longer incubation period of 6 hours at 37°C. This extended incubation is critical for the germination of anaerobic spores and for identifying slow-growing organisms such as C. acnes (internal data).
To effectively detect stressed microbial flora and spores, a specific protocol involving an incubation phase has been developed (Figure 5), aligned with the requirements of Chapter 2.6.27 of the European Pharmacopoeia. For the detection of aerobic microorganisms, including yeasts and molds, a 4-hour incubation is essential to ensure spore germination and the activation of sufficient metabolic activity. In contrast, detecting anaerobic microorganisms necessitates a longer incubation period of 6 hours at 37°C. This extended incubation is critical for the germination of anaerobic spores and for identifying slow-growing organisms such as C. acnes (internal data).
Level 3: Four-Day Sterility Testing
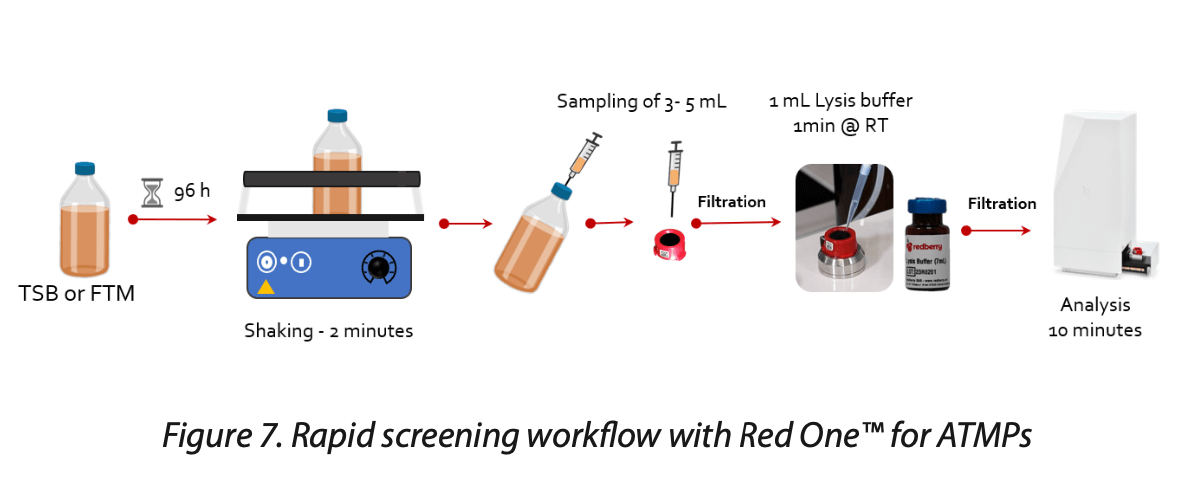
For sterility testing, Pharmacopeia guidelines[1] require a detection limit of 1 CFU. Achieving this level of sensitivity requires an incubation phase to promote the growth of any potential contaminants. Traditionally, the compendial method necessitates a 14-day incubation period to visually detect contamination. However, the Red OneTM system, with its higher sensitivity compared to visual detection methods, can reduce the time-to-result to as little as 96 hours.
In the context of CGT analysis, after the incubation period in a bottle, a sample is withdrawn, subjected to lysis, and then analyzed using the Red OneTM system (Figure 7). The lysis step effectively reduces background noise to below the system’s detection threshold without compromising the detection of microorganisms. This approach allows for the detection of contamination levels as low as 1 CFU within CGT products in just 4 days, including slow-growing microorganisms such as Cutibacterium acnes.
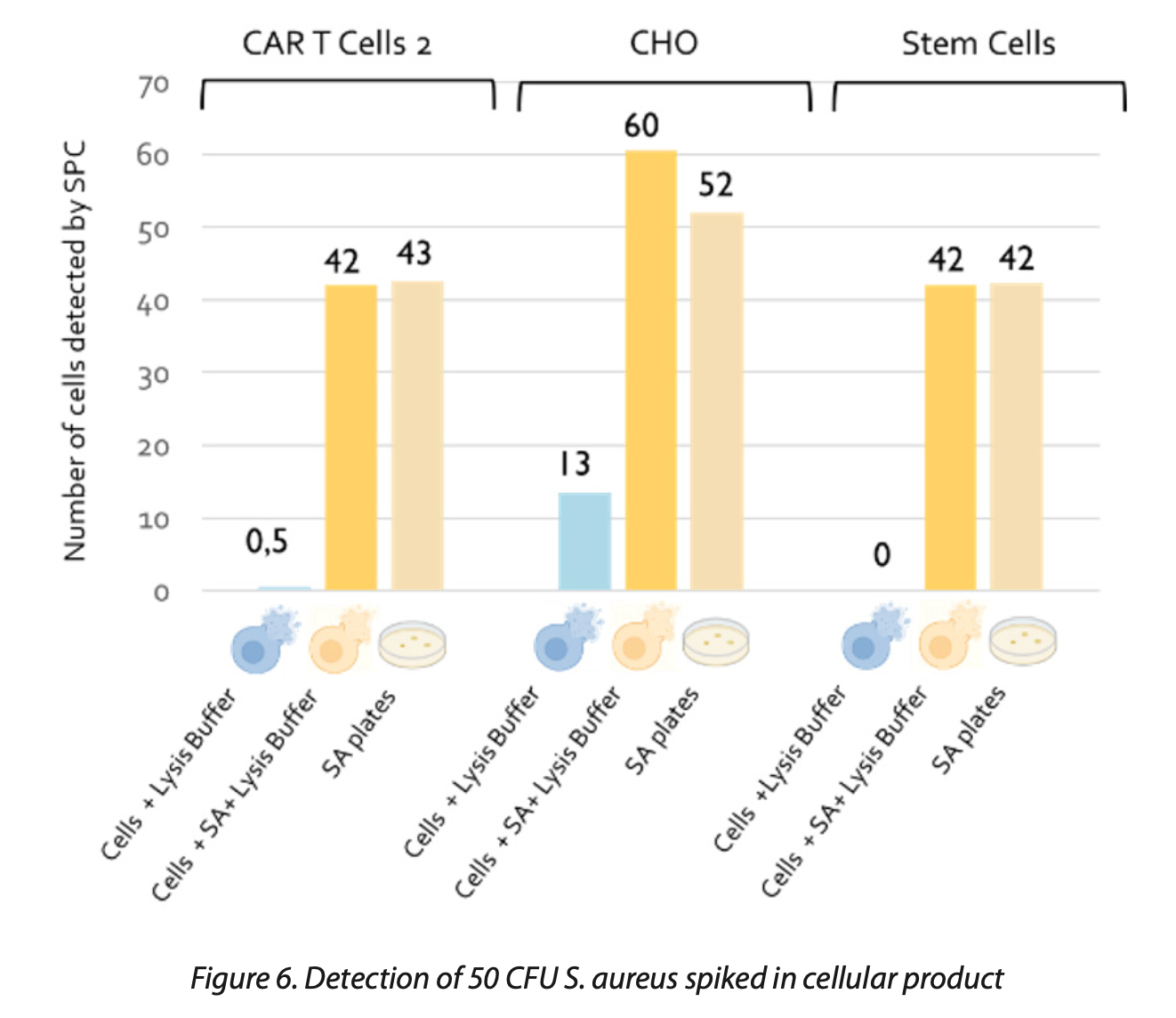
As shown in Figure 8, after a 4-day incubation period followed by the lysis step, mammalian cells (e.g., CAR T cells) were not detectable by SPC. This outcome demonstrates that the lysis treatment effectively removes these cells from detection. In contrast, after the lysis process, S. aureus was reliably detected by SPC. This finding highlights the technique’s effectiveness in identifying very low levels of microbial contamination, down to 1 CFU, within. CGT products.
The initial validation of a similar protocol, conducted in accordance with CY the Eur. Ph. 5.1.6 guidelines, was initiated for filterable pharmaceutical products incubated in canisters and is expected to be completed byCMY the end of 2024. Preliminary findings indicate that the limit of detection K (LOD) for this rapid method is comparable to, if not better than, the detection limit of the compendial method for the six microorganisms recommended by the pharmacopoeia, including C. acnes. Given its slow growth characteristics, C. acnes is particularly critical for this assay as it determines the turnaround time for obtaining results.
This initial validation has shown that the performance of the Red OneTM system meets or exceeds the required detection sensitivity, ensuring reliable and accurate results in compliance with established pharmacopeial standards.
4. Conclusion
The Red OneTM automated system offers a practical and effective solution for the rapid detection of microbial contamination in cell and gene therapy products. By employing solid phase cytometry combined with a selective lysis reagent, the system effectively addresses the challenges associated with detecting low levels of microbial contamination in the presence of high concentrations of mammalian cells.
This feasibility study has highlighted the potential of the Red OneTM system across three distinct workflows, each designed to meet specific requirements for contamination risk management:
• a 10-minute analysis for the immediate assessment of significant contamination, particularly suitable for time- sensitive situations where rapid decision-making is essential,
• a 6-hour analysis compliant with pharmacopoeia standards with a sensitivity of 100 CFU,
• a 4-day sterility test as defined in the pharmacopoeia, capable of detecting 1 CFU.
Overall, the Red OneTM system shows promise in enhancing the efficiency of microbial testing in CGT products by providing reliable results within a reduced timeframe. As CGT products continue to develop, rapid and accurate microbiological control will remain essential for ensuring product safety and efficacy.
Share


References
1. European Pharmacopoeia Commission. “Chapter 2.6.1. Sterility” in European Pharmacopoeia 7th ed.; Council of Europe: Strasbourg, France, 2010.
2. European Pharmacopoeia Commission. “Chapter 2.6.27.” in European Pharmacopoeia 7th ed.; Council of Europe: Strasbourg, France, 2010.
3. European Pharmacopoeia Commission. “Chapter 5.1.6. Validation of Alternative Microbiological Methods” in European Pharmacopoeia 7th ed.; Council of Europe: Strasbourg, France, 2010.