Sommaire
- Gene therapy manufacturing comes of age: Commercial-scale manufacturing is imminent. Are gene therapy innovators ready?
- Overcoming obstacles in downstream bioprocessing of AAV based gene therapy products
- Overcoming challenges in the development of lentiviral vector manufacturing platforms
- Are modern scalable bioreactors the Cell Culture Strategy needed for Gene & Cell Therapy success?
- The magnetic power of nanoparticles magnetic cell sorting decontamination of-environments and reusable nanocatalysts
- Development of a new preventive approach to reduce microbial infections with metal oxide nanoparticles
- Maximizing Sterility Assurance: Sterile Hold Time Testing for Sterilized Items Used in Parenteral Drug Manufacturing
The magnetic power of nanoparticles: magnetic cell sorting, decontamination of environments and reusable nanocatalysts
Nanotechnologies are based on the knowledge of the infinitely small allowing the creation of new properties and fundamentally innovative functions. The world of nanoparticles therefore represents a multidisciplinary research and development field with considerable potential regarding applications [1][2].

Nanoparticles are materials composed of grains with at least one of the three external dimensions measuring between 1 and 100 nm[1]. Their small size gives them revolutionary properties in terms of reactivity: the smaller their size, the larger their specific surface area relative to their volume and the greater the increase in surface reactivity. And the more reactive a particle, the greater its utility in terms of applications. Therefore, nanoparticles are used in a wide range of innovative applications such the agri-food industry, the field of building and public works, cosmetics, hygiene products, energy, the environment, paints, medicines, plastics processing or textiles for example[3]. Because of their diverse physical and chemical properties (large specific surface area, magnetic properties ), SPIO or “Superparamagnetic Iron Oxide”, are iron oxide nanoparticles used for industrial or biological applications, such as catalysis, nanomedicine or decontamination. These magnetic nanoparticles are prepared by the SON startup, which specializes in the development and manufacture of nano-objects (nanoparticles with or without functionalization). They are therefore biocompatible, reproducible and highly characterized via numerous techniques (X-ray diffraction (XRD), Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectroscopy (XPS), infrared analysis, X-ray fluorescence, Dynamic light scattering, zetametry, BET specific surface area measurement, thermogravimetric analysis…).
1. The magnetism of iron oxide nanoparticles
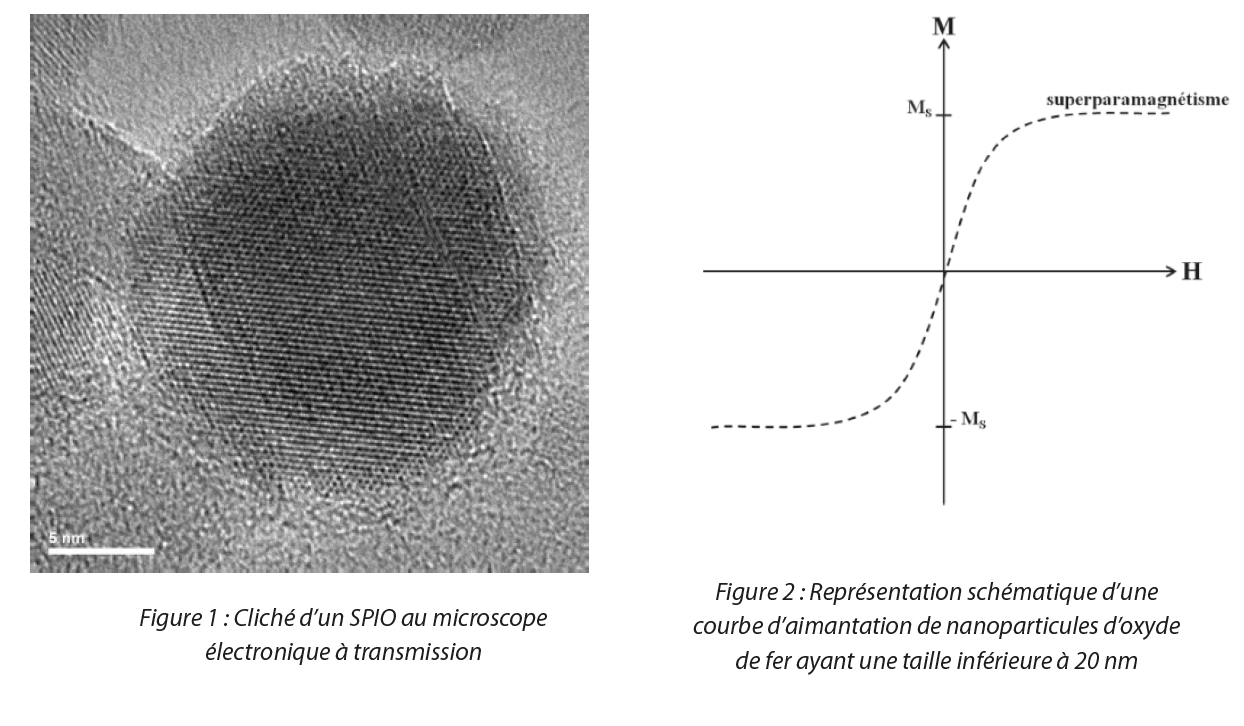
SPIO are iron oxide nanoparticles with particularly valuable properties. Their specific structure and the size of their crystallites, below 20 nm (Figure 1), give them a superparamagnetic property at ambient temperature[4]. In other words, when a magnetic field is applied, magnetization of the nanoparticles occurs. However, in the absence of a magnetic field, the magnetization of these nanoparticles disappears, as illustrated in Figure 2. Additionally, the more intense the field, the more intense is the magnetization also. This superparamagnetism is therefore a property that may prove relevant in some applications and vital particularly for biological applications as remanent magnetization (when the magnetic field is absent, the object retains its magnetic properties) is responsible for particle agglomeration resulting in the blockage of blood circulation in the vessels [5].
1. The crystallographic structure of SPIO
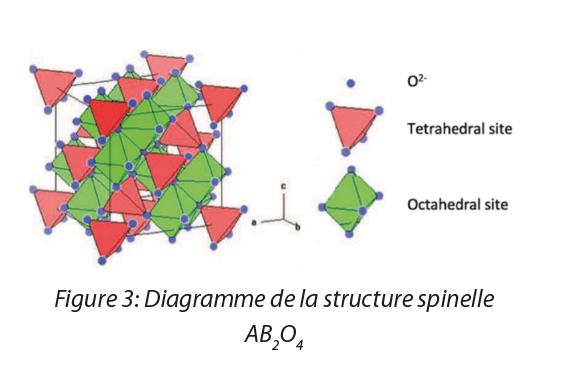
Magnetic iron oxide nanoparticles have an inverse spinel structure (Fe3+)A(Fe3+Fe2+¢)BO4 which is organized in the following manner:
- The 32 O2- anions constitute a face-centered cubic lattice (O);
- The Fe3+ ferric ions that are lodged in the tetrahedral sites (A) of the lattice, occupying 8 of the 64 tetrahedral sites;
- The Fe3+ ferric ions, Fe2+ferrous ions and the vacancies ¢ which are lodged in the octahedral sites of the lattice (B), occupying 16 of the 32 available octahedral sites.
Two types of iron oxide have this structure: magnetite ((Fe3+)A(Fe3+Fe2+)BO4)) and maghemite (obtained by oxidation of magnetite) ((Fe3+)A(Fe3+¢)BO4)). SPIO have an intermediate structure between these two oxides, but which tends strongly towards magnetite. It is therefore this specific structure which gives iron oxide nanoparticles their specific magnetic properties.
2. The toxicity of iron oxide nanoparticles
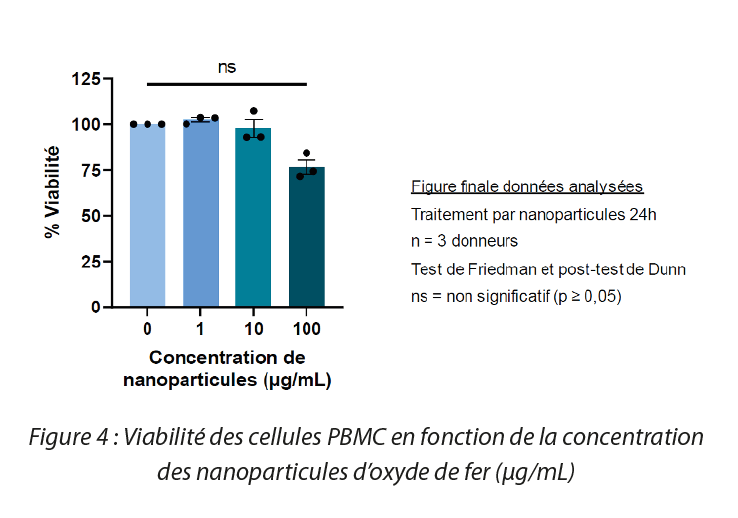
In order to study cell viability in the presence of iron oxide nanoparticles, peripheral blood mononuclear cells (PBMC) were treated with solutions of various concentrations of SPIO for 24 hr at 37°C. After these 24 hours of treatment, an MTT assay was performed. In this way it was demonstrated that SPIO do not impact cell viability in a statistically significant manner. However at higher concentrations (100 μg/mL), decreased viability is observed.
2. Some examples of applications of nanoparticle magnetism across different fields
a. Magnetic sorting of biological materials
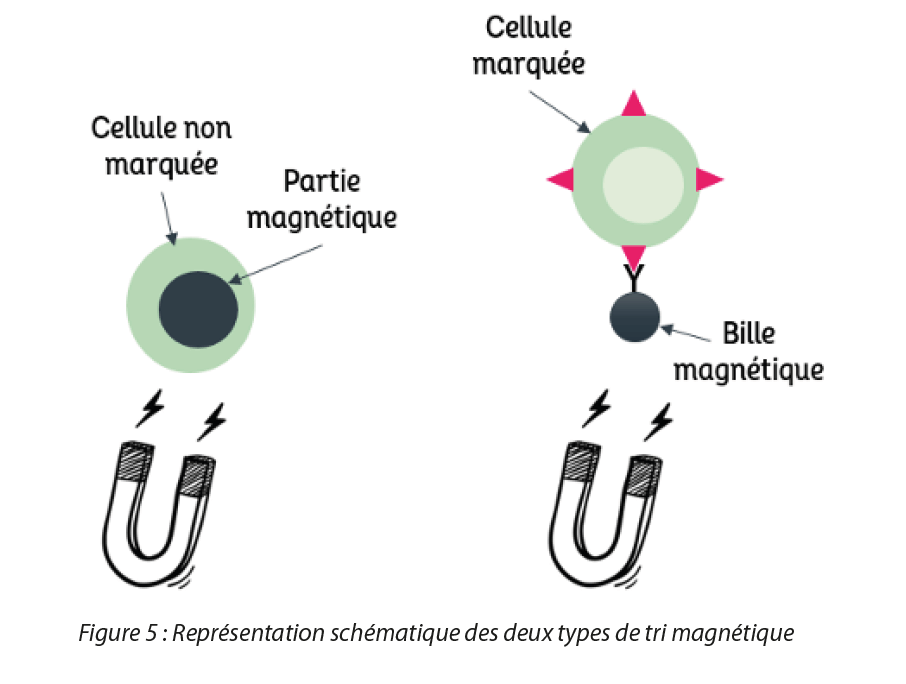
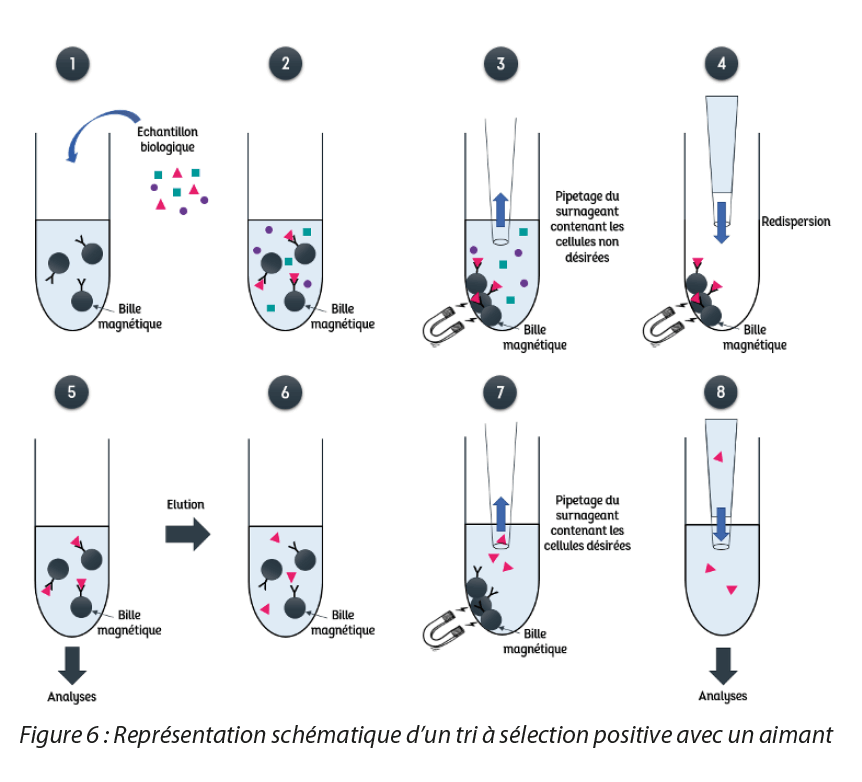
Magnetic cell sorting allows the selection or removal of different types of biological components such as cell types, intracellular organelles, proteins, or nucleic acids for example. This technique has numerous advantages such as the direct isolation of cells obtained from untreated samples, such as blood or bone marrow. It selects molecules simply and rapidly, particularly with the recent automation of this method. Compared to sorting by flow cytometry, another technique used to select subpopulations of cells, magnetic sorting eliminates the problem of interference with the movement of ions and carries out the selection of a large population of viable cells more rapidly [6]. There are two types of magnetic cell sorting, illustrated in Figure 3. The first consists in the separation of cells that already contain magnetic substances (e.g.: erythrocytes which contain a magnetic substance within the hemoglobin): these cells can thus be attracted directly by a magnet. The second involves the coupling of magnetic particles with a ligand capable of specifically recognizing a cell surface structure: a magnetic bead coupled to an antibody for example, will be able to bind to a cell displaying specific markers that will be able to recognize the antibody so forming an assembly capable of being attracted by a magnet. Then, two types of selection are possible. Positive selection consists in recovering the cells of interest magnetically. However, in the case of the second type of sorting presented above, when the ligand used risks modifying the cellular function of the targeted cell or else no ligand exists to recognize this cell, it is possible to carry out negative selection: the cells recovered by the magnetic method are the unwanted cells. Different methods are used to carry out magnetic cell sorting, such as column-based sorting[6] or sorting carried out directly with a magnet.
The latter method will be presented in the rest of the article and is illustrated by Figure 4 for the case of positive selection. First of all, magnetic particles are coupled to a ligand capable of recognizing the cell type to be selected. Then, the biological mixture is placed in solution with the coupled magnetic particles (1).
The ligand specifically recognizes the cell type to be isolated and binds to these cells(2). When a magnet is applied, the cells to which the magnetic particles are attached will be attracted towards the magnet. It is then possible to pipette off the supernatant containing the subpopulations of unwanted cells(3). Once the pipetting has been performed, the isolated cells can be re-dispersed(4). The cells can thus be analyzed directly and used(5), despite the presence of magnetic particles on their surface. In fact, these particles are much smaller than the eukaryotic cells, enabling them to limit cellular stress. The smaller the particle size, the greater the reduction in cellular stress, hence the use of nanoparticles which minimize this effect. In addition, in most cases, neither the function, viability, or use of cells are affected by the magnetic particles[6]. However, it is possible to elute these cells labeled by a magnetic agent and separate the latter using an enzymatic or physical method. Finally, to isolate cells from the magnetic particles, it is enough to perform magnetic purification, as previously described, with the presence of a magnet(6 to 8). In this way, this magnetic cell sorting technique allows over 95% cell purity to be obtained[6].
b. Removing heavy metal pollution from waste water
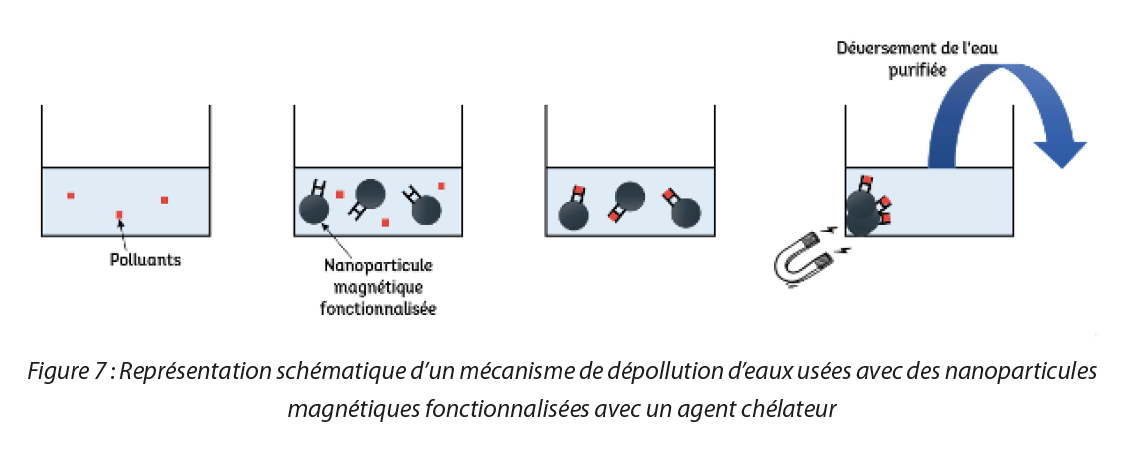
Many heavy metals such as mercury (Hg), lead (Pb), cadmium (Cd), but also, copper (Cu), nickel (Ni), chromium (Cr), arsenic (As), cobalt (Co), zinc (Zn) or manganese (Mn) for example are continually released into the environment mainly through human activities (waste from metallurgic, textile factories or paper mills for example, ground application, incineration of waste and combustion of gasoline,…). However, these heavy metals, which are not biodegradable, induce a certain toxicity for the environment and living beings. Several methods have been developed to decontaminate environments. One of these involves the use of chelating agents. Chelating agents more commonly called “chelators” are capable of capturing the heavy metals present in waste water via a complexation mechanism. These chelators have a specific affinity for each metal. In other words, each chelator possesses a greater or lesser affinity for the chelation of a specific metal. The value of grafting these molecules onto the surface of an SPIO is to be able to recover the assembly of nanoparticles functionalized by the chelators, which have themselves trapped the metal to be removed, by magnetization. Thus, as illustrated in Figure 5, it is enough to introduce the nanoparticles functionalized with chelators into an environment polluted by heavy metals. Then, the chelators on the surface of the nanoparticles are able to trap one or more metals. Then, all that is required is a magnet to recover the whole, since the nanoparticles, which have an iron oxide core, are magnetic. In this way the metals are recovered in a single operation, rapidly and easily.
c. Reusable nanocatalysts
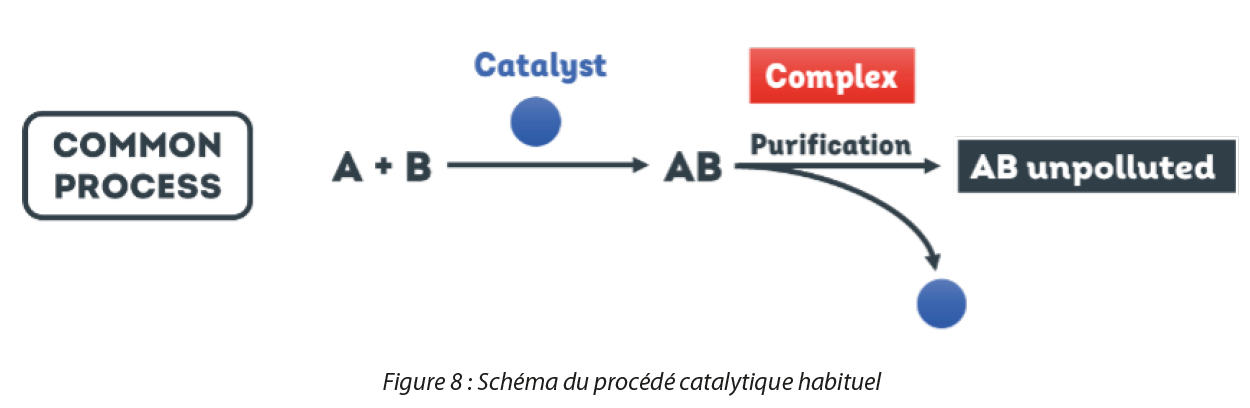
A catalyst is a key ingredient in numerous chemical reactions. It facilitates the transformation of one molecule into another molecule, without being transformed itself. 90% of chemical transformations in an industrial environment make use of metal catalysts for the synthesis of chemical products in large quantities. However, many catalysts are manufactured from precious metals, which makes them extremely costly and potentially hazardous for the environment. In parallel, as shown in Figure 6, following a reaction with a catalyst, manufacturers are required to carry out arduous purification steps energetically and economically in order to obtain their final product. Recovery of the catalyst in conventional processes is also difficult, which raises the problem of recycling and of the non-reuse of critical metals which could still theoretically be re-enlisted in a new catalytic reaction.
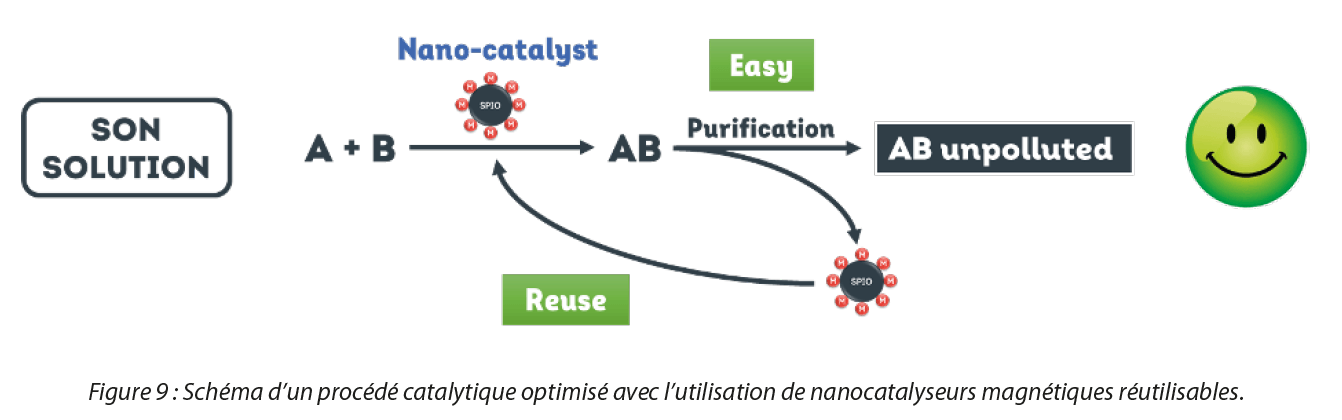
A new solution is seeing the light of day: reusable nanocatalysts. The basic technology relies on the coupling of magnetic nanoparticles, with catalytic metals such as palladium, ruthenium, rhodium, copper, nickel, gold, platinum or manganese on their surface,. The metals are attached directly to the surface, in a single synthesis step and without ligands contrary to the state of the art. By virtue of their magnetic core, it is thus possible to recover these nanocatalysts easily by magnetization in order to extend their life span, reusing them for up to 10 cycles in a new catalytic batch, as illustrated in Figure 7. Therefore, by recovering them and reusing them, manufacturers can reduce their production costs and opt for a more sustainable chemistry.
In addition, these new nanocatalysts offer other advantages such as the procurement of better catalytic performance because of their small size which gives them a very large reactive surface area. Indeed, a study comparing the catalytic performance of SON nanocatalysts (e.g. Palladium nanocatalyst) and conventional catalysts (e.g.: Palladium on carbon) was carried out. It was shown that for the same catalytic reaction, under similar conditions and with the same equipment as the manufacturers, the quantity of critical metal used (0.1% palladium immobilized on the nanocatalysts vs. 10% palladium in the palladium on carbon) and production costs (90% reduction in costs) were drastically reduced for the procurement of a higher yield (98% with nanocatalysts vs. 70% with palladium on carbon).
This nanotechnological innovation thus allows:
- Easy purification of the medium by magnetization in the presence of a magnetic device;
- Easy recovery of the nanoparticles by magnetization (or their isolation at the end of the reaction in the same tank);
- Reuse in a new catalytic batch; achievement of high-performance catalysis with a reactive surface area much larger than in conventional catalysis;
- Considerable reduction of production costs and choice of a less polluting and therefore more sustainable chemistry.
Therefore, the fields of application of nanocatalysts are vast. The latter may be used for different types of catalysis (e.g. Heck, Suzuki, Sonogashira, Ullmann), as well as for the dehydrogenation of amine-boranes to produce hydrogen[7].
 |  |
3. Conclusion
Superparamagnetic nanoparticles provide innovation in numerous sectors. In the medical field, magnetic nanoparticles represent a simple, rapid means of isolating a cell population. By being functionalized by chelating agents, they also allow the selective decontamination of environments. Finally, they represent an innovative solution for increasing catalytic performance, while progressing towards a greener and more sustainable chemistry by allowing the reuse of critical metals.
Glossary
SPIO (SuperParamagnetic Iron Oxide): nanoparticles of iron oxide with superparamagnetic properties
Crystallite : portion of matter which has the same structure as a single crystal i.e. its structure is formed solely by a single family of atomic planes.
Superparamagnetic object: object that displays magnetic properties when it is placed in a magnetic field but which does not display any residual magnetism once removed from this field.
References
[1] Myriam RICAUD; Olivier WITSCHGER (INRS). (2012). Les nanomatériaux : Définitions, risques toxicologiques, caractérisation de l’exposition professionnelle et mesures de prévention (Edition INRS ED 6050). ISBN 978-2-7389- 20210-2. 14110 Condé-sur-Noireau: Corlet.
[2] Julien HACCOUN, Didier THERON, Aline TOURNIER. (2021). Les nanotechnologies : un nouveau paradigme, Les cahiers de l’ANR – n°5. Repéré à l’URL : https://anr.fr/fileadmin/documents/2012/Cahier-ANR-5-nanotechnologies. pdf
[3] Myriam RICAUD, Cécile OILLIC-TISSIER, Pascale BARBILLON, Catherine BRUGNOT, Christine DOLLE, Laurent FINA, Philippe LEBON, Frédéric MAITRE, Jean-Michel ODOIT (2014). Aide au repérage des nanomatériaux en entreprise (Edition ED 6174). ISBN 978-2-7389-2132-1.
[4] J.PARIS. (2015). Nanoparticules d’oxyde de fer et nanotubes de titanate pour l’imagerie multimodale et à destination de la thérapie cancéreuse. (Thèse de doctorat). Laboratoire Interdisciplinaire Carnot de Bourgogne, Université de Bourgogne, DIJON.
[5] C.J.EID (2010). Synthèse et caractérisation de nouvelles nanostructures à base d’oxyde et de carbure de Fe. (Thèse de doctorat en cotutelle). Université Claude Bernard, LYON. NNT : 2010LYO10172. Tel-00720870.
[6] F. Ghiringhelli, E. Schmitt . Tri par billes magnétiques Technique et exemple du tri des lymphocytes T régulateurs CD25+ chez le rat . Annales de Biologie Clinique. 2004;62(1):73-78.
[7] Poinsot D., Bouzid M.; Burlot A. ; Mboyi C. D. ; Doulain P. E. ; Paris J. ; Heintz O. ; Domenichini B. ; Colliere V. ; Kahn M. L. ; and Hierso J.-C.. High Recyclability Magnetic Iron Oxide‐Supported Ruthenium Nanocatalyst for H2 Release from Ammonia‐Borane Solvolysis. ChemNanoMat, 2022, 9 (8), pp.e202200285. (10.1002/cnma.202200285). (hal-03728542)
Share