October 2024
La Vague n°83
EU GMP Annex 1 / Patient risk management ICH Q9(R1) / Digitization
Summary
- What does 21 CFR Part 11 mean in everyday online analytics?
- Digitalization in cleaning validation an overview of possibilities, challenges, and opportunities for savings?
- EU GMP Annex 1. Implementation of Contamination Control Strategy
- Impact of the new Annex 1 on Sterile Filling
- The Challenges of Floor Cleaning & Sanitization
- Réduction énergétique des centrales de traitement d’air : comment adapter son monitoring environnemental ?
- USP <922> Water Activity: A Better Approach for Lyo Moisture Determination. Applications enabled by rapid non-destructive headspace moisture analysis of freeze-dried product
- Sustainable water management in the pharmaceutical industry
- Rapid Testing for Cell & Gene Therapy Products: A Three-Level Approach Using an Automated Solid Phase Cytometry System
USP <922> Water Activity: A Better Approach for Lyo Moisture Determination. Applications enabled by rapid non-destructive headspace moisture analysis of freeze-dried product
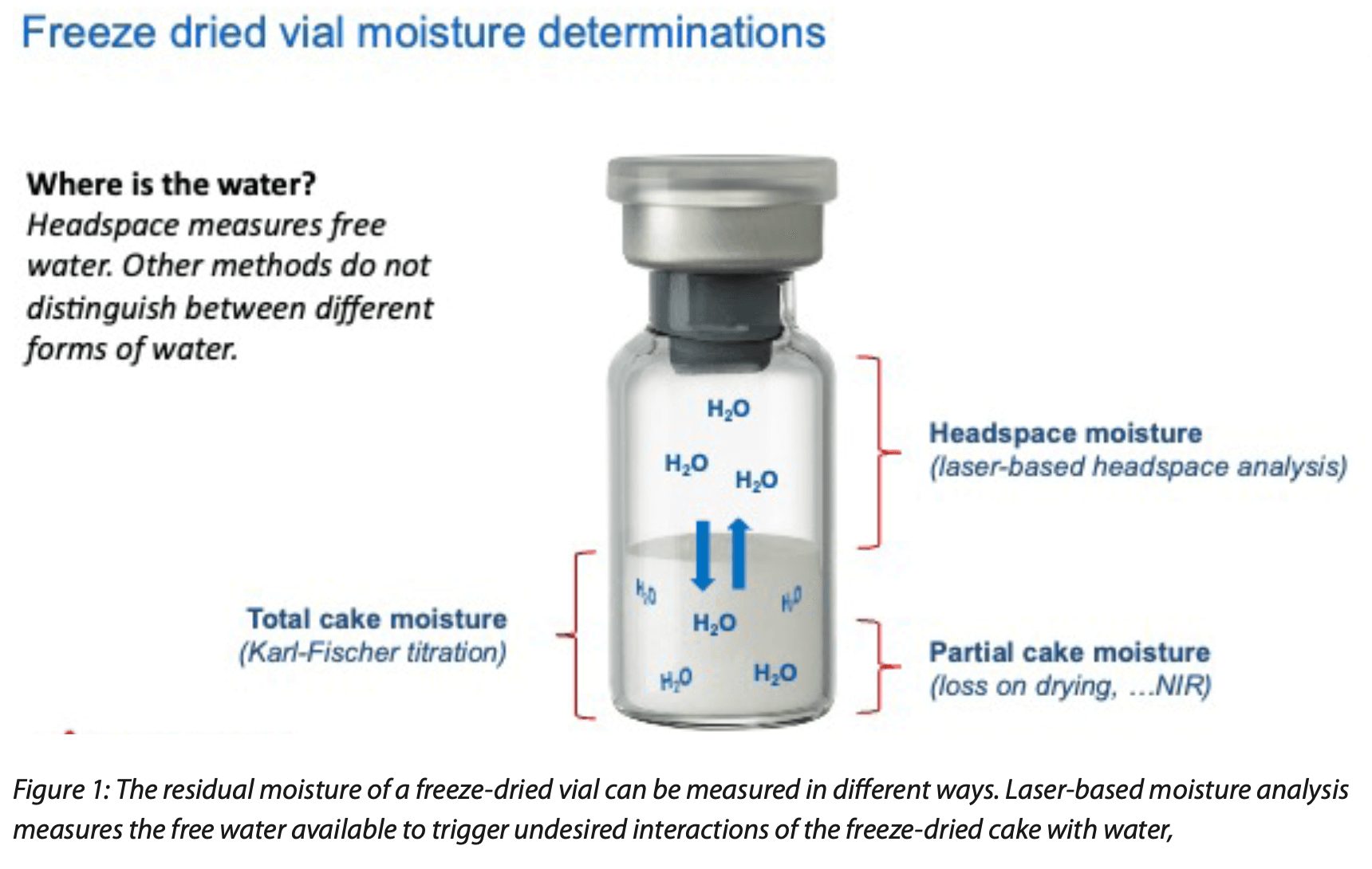
Residual moisture content is a critical quality parameter for freeze-dried pharmaceutical product and exists in different forms. Only the relatively “free”, in contrast to the more “bound”, water is capable of inducing degradation events within the product. However, for historical reasons, the global pharmaceutical industry standardized on total water content as the primary moisture quality parameter. The traditional moisture analysis techniques used by the industry, gravimetric or Karl Fischer (KF) titration methods, cannot distinguish between the different water states.
Most other industries (for example, the food industry) have standardized on a measurement of water activity as the primary moisture quality parameter. Scientific investigation has concluded that water activity is a far better predictor of product safety and stability than Karl Fischer water content. This is because water activity is a thermodynamic property giving the potential for the product to interact with water and it directly correlates to moisture dependent properties of the product. For this reason, the US Pharmacopeia has recently implemented a new chapter, USP <922> Water Activity, to stimulate more use of water activity as a science-based moisture quality parameter. This article motivates why the pharmaceutical industry needs to evolve the total moisture content quality paradigm currently being used towards water activity. Concrete industry case studies are presented as examples of how non-destructive laser-based headspace water activity methods can be used to analyze moisture content of freeze-dried pharmaceutical product. Laser-based headspace water activity methods enable the straightforward generation of statistical moisture data for lyo cycle optimization, lyo chamber moisture mapping, and batch moisture characterization. These case studies were presented at a workshop given at the A3P Lyophilization 2023 Conference in Lyon and in a recent book chapter publication[1].
1. Importance of residual moisture determination
Determining the residual moisture content of a lyophilized pharmaceutical product is important for several reasons. First, the amount of residual moisture content is related to the stability of the formulation over the product shelf life. Small-molecule formulations can have direct degradation pathways triggered by water, and it is crucial that all finished product is dried to a level below
a defined residual moisture specification. In general, the degradation pathways for large-molecule formulations are more complex, with water often playing an indirect role. It should be noted here that the residual water content in a freeze-dried product is present in a number of different states that are differentiated by the intensity and type of its chemical and physical interactions with both the excipients and the active pharmaceutical ingredient (API). At the most simplistic level, these different states are frequently referred to as either “bound” – including adsorbed, chemically bound and water of crystallization – or “free” to denote water that, in contrast, may be weakly interacting with the solutes and is free to migrate based on the chemical potential between components of the formulations and the environment surrounding the product. It is the scientific consensus that only this free, or active water, is available for chemical reactions. Because too much free water can trigger possible degradation pathways of the active pharmaceutical ingredient, the presence of free water has the greatest impact on product stability.
A second reason underlying the importance of residual moisture determination is that process studies involving residual moisture analysis of finished product can give deep insight into the freeze-drying process itself. In particular, the consequences of process variability on final product quality can be investigated and characterized. Residual moisture determination can be used as a tool to confirm the efficiency and robustness of a specific freeze-drying cycle for a particular drug formulation, or to optimize and validate the performance of a particular freeze dryer. Typical pharmaceutical freeze-drying cycles target residual moisture contents in the range of 1% to 3% water by weight. Historically, a strategy that can be described as “the drier, the better” was often followed. For small molecules having a direct degradation pathway triggered by water, this approach was an appropriate strategy. However, in the world of large biopharmaceutical molecules, it is possible to over-dry the product and control of the process as well as analysis of final product becomes more critical.
It is now generally known that even in the lyophilized state, proteins depend on small quantities of water to help maintain higher-order structure. Other types of products, such as certain lyophilized blood plasma formulations, need a minimum amount of water to achieve efficient dry-heat viral inactivation. It is therefore sometimes necessary to design a freeze-drying cycle that keeps all finished product samples within a certain moisture range, having both minimum and maximum specifications. During ongoing pharmaceutical industry operations, research and development efforts often do not have the time to fully optimize the formulation and/or the freeze-drying cycle. Although conservative drying cycles produce product that meet quality parameters (i.e., sufficiently low residual moisture), the same product quality could possibly be produced with shorter cycles, saving time and energy costs, if appropriate development is done to optimize the formulation and the process.
2. The need for a better residual moisture method
In contrast to the focused attention and innovative work in the past years to design and develop improved freeze-drying processes, less progress has been made in developing better residual moisture determination techniques for the analysis of finished product. The traditional methods used in the pharmaceutical industry for residual moisture determination have been thermogravimetric analysis (TGA) and Karl Fischer (KF) titration. For freeze-dried product, Karl Fischer titration is the most widely used method today and measures the total water in the freeze-dried cake, assuming that the sample is wholly soluble in the KF medium. Although titration methods have improved over the years, KF methods are time-consuming, require operator expertise and careful sample handling, and are destructive (the sample is destroyed during the analysis). Unlike Karl Fischer, which relies on a chemical reaction to detect water, TGA methods measure the weight loss as the sample is heated, driving off residual moisture.
These methods measure not only the water but also any other volatiles that are produced as a result of heating. Therefore, the composition of the lyophilized material must be well understood for TGA methods to be used for accurate measurement of the residual water content. The methods also suffer from potential artifacts due to environmental moisture and therefore require careful handling by the operator, are time-consuming, and are also destructive.
The destructive nature of the traditional methods limits their utility for generating statistically relevant data in freeze-dried product stability and process studies. In a stability study where moisture content is to be correlated to the concentration of active ingredient, a set of vials must be destructively tested at each time point for each parameter. Therefore, sample sets are kept relatively small to avoid destruction of scarce material. In addition, an implicit assumption is made that each of the vials tested at a particular time point are identical, which may or may not be the case. The ability to measure both product moisture and degradation of active ingredient in a single sample would result in more robust data to determine accurate moisture stability specifications and require less material for a stability study. In the area of process studies, the absence of statistically relevant residual moisture data using the traditional methods means that limited insight can be gained into the consequence of process variability on final product quality.
A complementary moisture determination method that is rapid, as well as nondestructive, would enable statistical moisture data collection in process studies and give insight into both the consequences of process variability on final product quality as well as the root cause of the process variability. The results of such studies could then be fed back into process design activities. Finally, as mentioned before the traditional moisture methods do not distinguish between free (active) water and bound water, making it difficult to scientifically investigate the correlation of different states of water to degradation and to gain insight into moisture dynamics that might occur in the freeze-dried matrix over time. Improving process design and increasing knowledge of moisture dynamics for freeze-dried product will result in a more streamlined and efficient production process saving time and resource and will lower the risk of batch issues.
Clearly, a better residual moisture measurement approach is needed to overcome the shortfalls and disadvantages of KF titration methods. Developments in tunable diode laser absorption spectroscopy (TDLAS) offer an alternative technique enabling rapid, non-destructive moisture analysis. Laser-based headspace analysis measures the water vapor partial pressure in the product container headspace, providing a measure of the thermodynamic activity of the “free” water in the pharmaceutical sample. The laser-based headspace analysis technique has been used in the global pharmaceutical industry for more than two decades. It is most well known as a deterministic container closure integrity (CCI) testing technology described in USP <1207> and as a technique that can be used to non-destructively monitor headspace oxygen content of oxygen-sensitive products. Recent scientific work has demonstrated that headspace moisture analysis can be used as a residual moisture method and, in fact, gives a measurement of water activity[2-4].
Figure 1 shows a cartoon of a freeze-dried vial and the different forms of water that can be found in the product sample. It is clear that a headspace moisture (water vapor) measurement measures the free water which can be directly related to the water activity of the freeze- dried cake.
3. USP <922> Water Activity: A better moisture paradigm
The introductory paragraph in USP <922> states the following[5]: “Total water content is an important quality attribute…However, water may be allocated in more than one compartment within these materials. Some of it may be tightly bound and not available to participate in chemical, biochemical, or physicochemical reactions (e.g., as hydrate salts), whereas some of the water may be more loosely bound and more freely available to participate in reactions… It is important to establish what fraction of the total water is contained in the latter category, and the determination of water activity (aw) provides this information.”
The chapter goes on to describe a number of different applications for water activity:
“The determination of aw aids in decisions during ingredient and product processes design, ingredient selection, packaging selection, and product storage. These include:
• Reducing the degradation of active ingredients within product formulations, especially those susceptible to chemical hydrolysis
•…
• Providing a complementary method to the Karl Fischer titration for monitoring changes in water content
• Controlling and monitoring physical, chemical, and microbial product stability
• …”
The USP <922> chapter then goes on to describe water activity instruments that can be used to analyze pharmaceutical product. The Optical Hygrometer – Tunable Diode Laser is an instrument that uses TDLAS to make a measurement of the partial water vapor pressure in a sealed sample which can then be related to the water activity at a defined temperature.
Existing Headspace Moisture Analyzers are considered to be an Optical Hygrometer and can be used to make a water activity measurement of freeze-dried product vials. As a rapid nondestructive measurement,
headspace moisture analysis can complement the traditional residual moisture determination methods to provide robust statistical residual moisture data. This results in a much-improved moisture quality paradigm enabling a variety of applications during the product life cycle of a freeze-dried pharmaceutical product that give deep insight into product and process quality.
As the following case studies will show, water activity not only gives the industry the possibility to more reliably be compliant to regulatory standards it also has helped drive innovation, improve scientific productivity, and shorten the drug pipeline at many of the top global pharmaceutical companies.
4. Industry Case Studies: Headspace Water Activity for Freeze Dried Vials
Industry Case Study 1
Headspace Water Activity Analysis as a Tool for Freeze Dryer Shelf Mapping, Cycle Optimization, and Demonstration of Freeze Dryer Equivalence
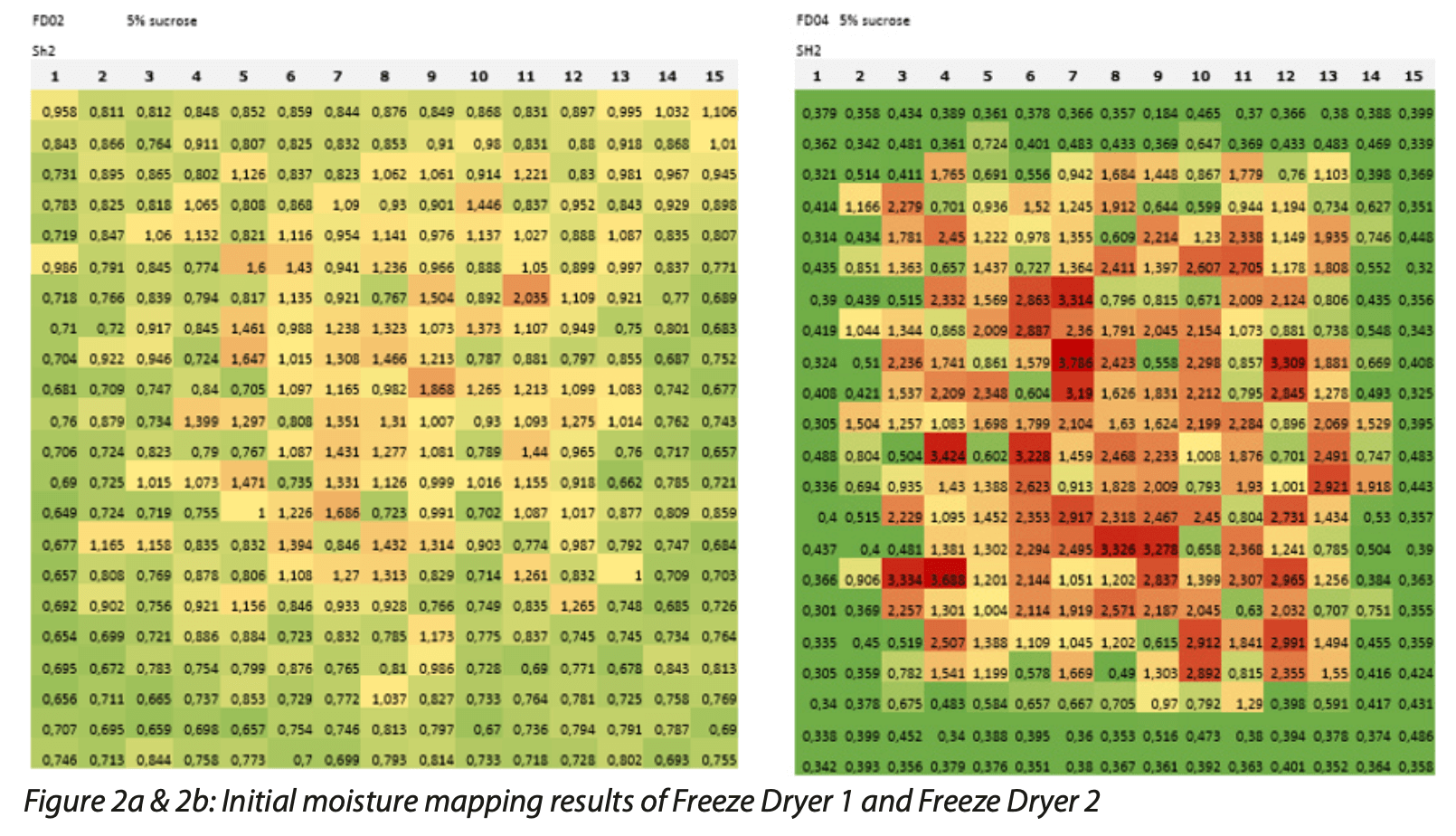
When scaling up the production of a commercial freeze-dried product over multiple freeze dryers at the same site, or across different sites, one of the challenges is to demonstrate that the freezer dryers are equivalent to one another. Duplicating the same freeze-drying cycle on different pieces of equipment often does not produce the same uniformity and end product moisture content values. With the limited final product testing that can be achieved with the traditional moisture determination methods, there is usually no data available giving deep insight into the impact of process and equipment variability across a full commercial scale batch. The objective in this study was to investigate if the two freeze dryers could produce equivalent batches with respect to product moisture content. Figure 2a shows the moisture map of one of the shelves in Freeze Dryer 1. Rapid nondestructive headspace moisture analysis was used to perform 100% analysis of a 5% sucrose placebo formulation that was freeze -dried with a defined cycle. The resulting moisture map shows signs of wetter product in the center of the shelf and the measured moisture values were slightly above the desired moisture content. Figure 2b shows a moisture map of the corresponding shelf in Freeze Dryer 2 and the results are much different. There is more variability in the measured water vapor levels across the shelf, with the edge vials having been dried to their desired moisture content but the center vials being much wetter. Clearly, the two freeze dryers are not equivalent when using the defined cycle to freeze dry the 5% sucrose placebo formulation.
Because the freeze dryers were to be used to lyophilize biopharmaceuticals, it was decided to add 2.5% bovine serum albumin (BSA) to the placebo formulation. Figure 3a & 3b show the moisture maps produced from these second batches produced with Freeze Dryer 1 and Freeze Dryer 2, respectively, by using rapid non- destructive headspace moisture analysis. After optimizing formulation and lyo cycle, the moisture maps now show end product that has extremely good uniformity across the shelf for both freeze dryers with moisture values that are all within the desired range.
This case study again demonstrates the utility of headspace moisture analysis as an analytical tool enabling deep insight into potential moisture content variability in the finished product due to the process, freeze drying equipment, and product formulation.
Industry Case Study 2
Correlation of Product Stability to Headspace Water Activity
The study described here demonstrates that the stability of a pharmaceutical freeze-dried product can be quantified in terms of a vial headspace water vapor determination (or, equivalently, water activity of the formulation) measured using laser-based headspace analysis. For the study, a pharmaceutical freeze-dried formulation that has well-characterized degradation pathways due to the presence of moisture was chosen. Product stability studies and subsequent data analysis showed that the headspace water vapor content in a sealed freeze-dried product vial directly correlated with the degradation rate of the pharmaceutical product.
Typical production samples contain approximately 0.5% water by weight (w/w) moisture, corresponding to a water content of approximately 1.1 mg/vial. Samples with a range of different moisture levels for the stability study were made with product moisture contents of 0.5%, 1.0%, 1.5%, 2.0%, and 3.0% (w/w).
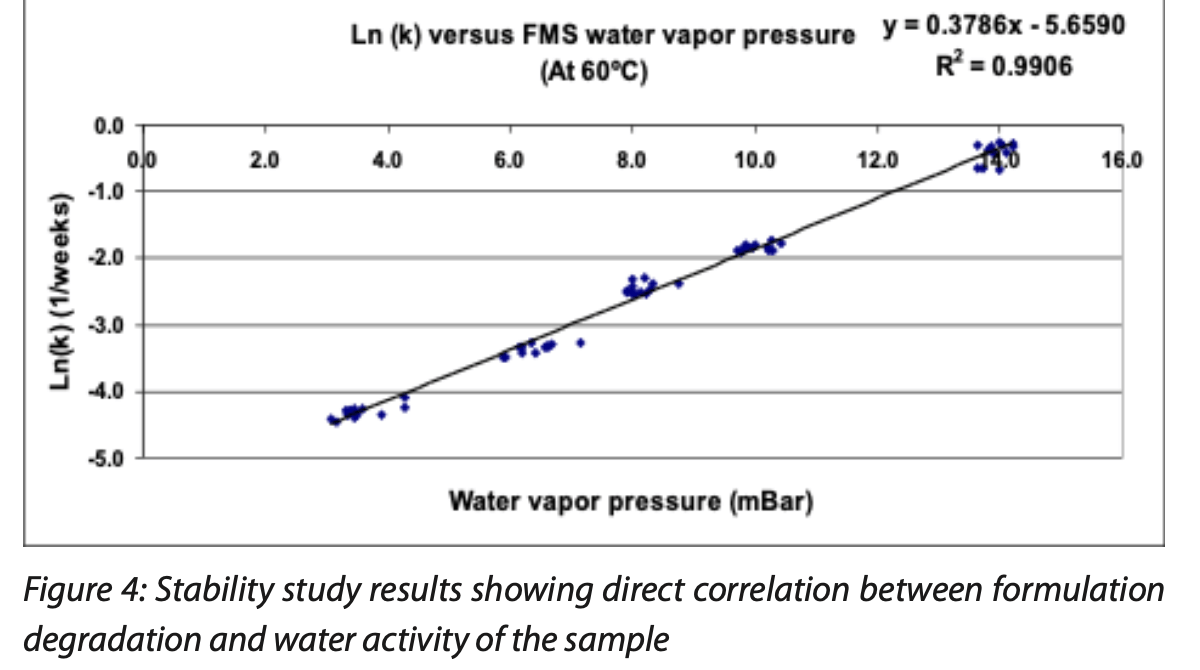
The product vial samples were divided into stability groups and stored at either 25°C, 40°C or 60°C. At defined time points, all samples were removed from their respective accelerated stability storage condition to nondestructively measure their headspace moisture content by using rapid nondestructive headspace moisture analysis. A subset of these samples was then destructively tested using both KF and HPLC techniques; the remaining samples were then place back into their respective storage conditions until the next scheduled time point. Using HPLC chromatography, the rate of decay of the pharmaceutical product was determined by making measurements at three time points for samples containing five different product moistures stored at three different temperatures. Figure 4 illustrates the correlation results for the samples stored at 60°C. The graph plots the log of the exponential degradation contant versus the partial water vapor pressure (water activity) measured in the sample.
These results show an excellent correlation between product stability of a freeze-dried formulation and the headspace water vapor pressure (water activity). In this residual moisture paradigm, a straightforward rapid nondestructive headspace moisture measurement very accurately predicts the product stability – KF titration and total moisture content measurements is not needed anymore.
Summary
Laser-based headspace moisture analysis is an analytical tool that has proven to be useful during product life cycle activities of pharmaceutical freeze- dried product as a residual moisture determination method. The rapid, nondestructive nature of the measurement technique means it is well-suited for analyzing large numbers of product samples. As a complementary tool to the traditional gravimetric and KF titration methods, which are relatively slow and destructive, headspace moisture analysis enables the generation of robust statistically relevant product moisture data needed in process development, stability studies, and commercial batch characterization. Applications include enabling the elucidation of water dynamics in lyophilized cakes, characterizing batch moisture uniformity, formulation and lyo cycle optimization, lyo chamber shelf moisture mapping, the demonstration of freeze dryer equivalence, and possible moisture inspection of product batches to identify random out-of-specification product vials. Finally, it has also been shown that headspace moisture measurements can be readily translated into the water activity of the product. As a fundamental thermodynamic property of the freeze-dried material, water activity determination can be used to establish the stability profile of the formulation with respect to moisture content. Once the stability profile has been established as a function of water activity, the stability of a freeze-dried product vial can be predicted with a rapid nondestructive headspace water vapor measurement. A water activity paradigm is scientifically stronger in predicting stability than a total water content paradigm. Using water activity instead of total water content to correlate to product degradation is recommended by the new USP <922> Water Activity chapter.”
Share

References
1. Book chapter “Laser-Based Headspace Moisture Analysis for Rapid Nondestructive Moisture Determination for Lyophilized Products”; AAPS Advances in the Pharmaceutical Sciences Series (AAPS, volume 59), Principles and Practices of Lyophilization in Product Development and Manufacturing, Feroz Jameel, Editor
2. R.P. Affleck, et al. “NIR & FMS Spectroscopy as Non-Invasive Methods for Moisture Assessment of Freeze-Dried Biologics”. J PharmSci (2022). https://doi.org/10.1016/j.xphs.2021.06.016
3. Y. Pu & Y Li. “Noninvasive Moisture Detection in Lyophilized Drug Product Using NIR Spectrometer and Headspace Moisture Analyzer.” J PharmSci (2022). https://doi.org/10.1016/j.xphs.2022.11.009
4. Sonje, Jayesh, et al. “Mannitol hemihydrate in lyophilized protein formulations: Impact of its dehydration during storage on sucrose crystallinity and protein stability.” Int J of Pharmaceutics 624 (2022). https://doi.org/10.1016/j.ijpharm.2022.121974
5. U.S. Pharmacopoeia. USP 44 <922>. Water Activity. United States Pharmacopoeial Convention, Inc.: Rockville, MD, 2021