ASEPTIC PROCESSING
Isolators & RABS
Lugar
Hotel SB Diagonal Zero, Barcelona

Formato
Conferencias, Talleres, Exposición, Networking
Compartir
Tras varios años de gestación y múltiples versiones se ha publicado el nuevo Anexo 1 de las GMP.
Las últimas versiones corroboran el énfasis en la reducción de la interacción humana para minimizar riesgos de contaminación. Ya no cabe duda.
En el corazón de los procesos asépticos, el uso de aisladores y tecnologías de barrera será esencial para garantizar la calidad del producto. Por tanto, a partir de la publicación, el caballo de batalla en cuanto a cumplimiento será la transformación de equipos y procesos existentes utilizando estas tecnologías.
En este evento queremos que los fabricantes que llevan tiempo empleando tecnologías de barrera compartan su experiencia. A su vez, trataremos de analizar tanto su utilización práctica como su implementación desde el punto de vista técnico y operativo.
27 de Octubre de 2022
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
• Basics of isolators• Air Handling Units
• integrated transfer systems, RTP Port and MTC
• DECOPulse® – integrated H2O2 Bio-decontaminationsystem”
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
• Basics of Isolators• Test process inside isolator
• Technical solution for sterility test isolator
• Plug & Test – easy move-in concept
• Modular Design
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
Formulario de inscripción
Inscríbase al evento A3P Aseptic Processing.
Precio: 500€
Exposición completa
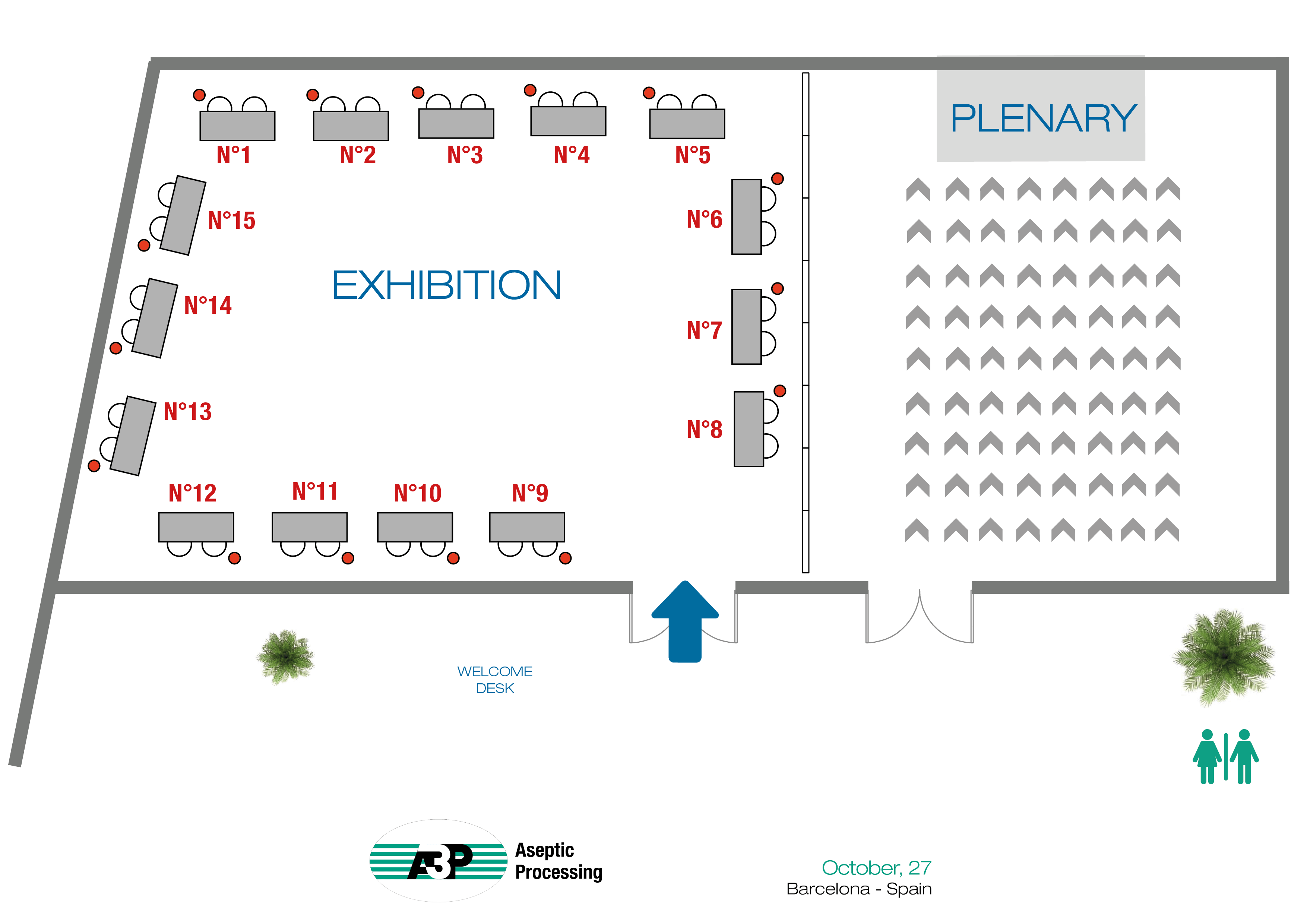
| Empresa | Table Top | Empresa | Table Top | |
| FPS PHARMA | 1 | MK-VERSUCHSANLAGEN UND LABORBEDARF e.K | 9 | |
| DEVEA | 2 | SALAMANDER U | 10 | |
| MESALABS | 3 | OPTIMA | 11 | |
| STERIS | 4 | BIOMERIEUX | 12 | |
| LITEK PHARMA | 5 | JACOMEX | 13 | |
| MARCHESINI | 6 | BECTON DICKINSON | 14 | |
| ECOLAB | 7 | JCE BIOTECHNOLOGY | 15 | |
| TISELAB | 8 |
Services included with the Table Top stand
- A dedicated exhibition area where you can install your products and communication tools: umbrella stand, roll-up, display, product, …
A basic equipment including a 180×80 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker - Breaks and meals as indicated in the program
- Illimited Wifi
- A badge that gives you free access to the entire technical and scientific programme.
- The list of participants to the event
Contact
Depending on your requirements and preferences, you can contact Florian Canuto:
Phone number: +33 (0)7 61 55 24 36
Email : fcanuto@a3pservices.com
How to access



