ASEPTIC PROCESSING
Isolators & RABS
Lieu
Hotel SB Diagonal Zero, Barcelona

Format
Conferences, workshops, Exhibition, Networking
Share
After several years of gestation and multiple versions, the new GMP Annex 1 has been published.
The latest versions corroborate the emphasis on reducing human interaction to minimise contamination risks. There is no longer any doubt.
In the heart of aseptic processes, the use of isolators and barrier technologies will be essential to ensure product quality.
Therefore, from the publication onwards, the compliance workhorse will be the transformation of existing equipment and processes using these technologies.
During this event we want manufacturers who have been using barrier technologies for some time to share their experience. In turn, we will try to analyse both their practical use and their implementation from a technical and operational point of view.
schedules and order of conferences may be subject to change
Our partners will offer you 3 workshops sessions during the day
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
• Basics of isolators
• Air Handling Units
• integrated transfer systems, RTP Port and MTC
• DECOPulse® – integrated H2O2 Bio-decontaminationsystem”
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
• Basics of Isolators• Test process inside isolator
• Technical solution for sterility test isolator
• Plug & Test – easy move-in concept
• Modular Design
The goal is to highlight the main new requirements of the “Annex 1” and evaluate their possible interpretation.
A basic guideline on “how to apply the new requirements on the filling lines” a few weeks after the release.”
Registration Form
Participate in A3P Aseptic Processing Event.
Price: 500€
The exhibition is complete
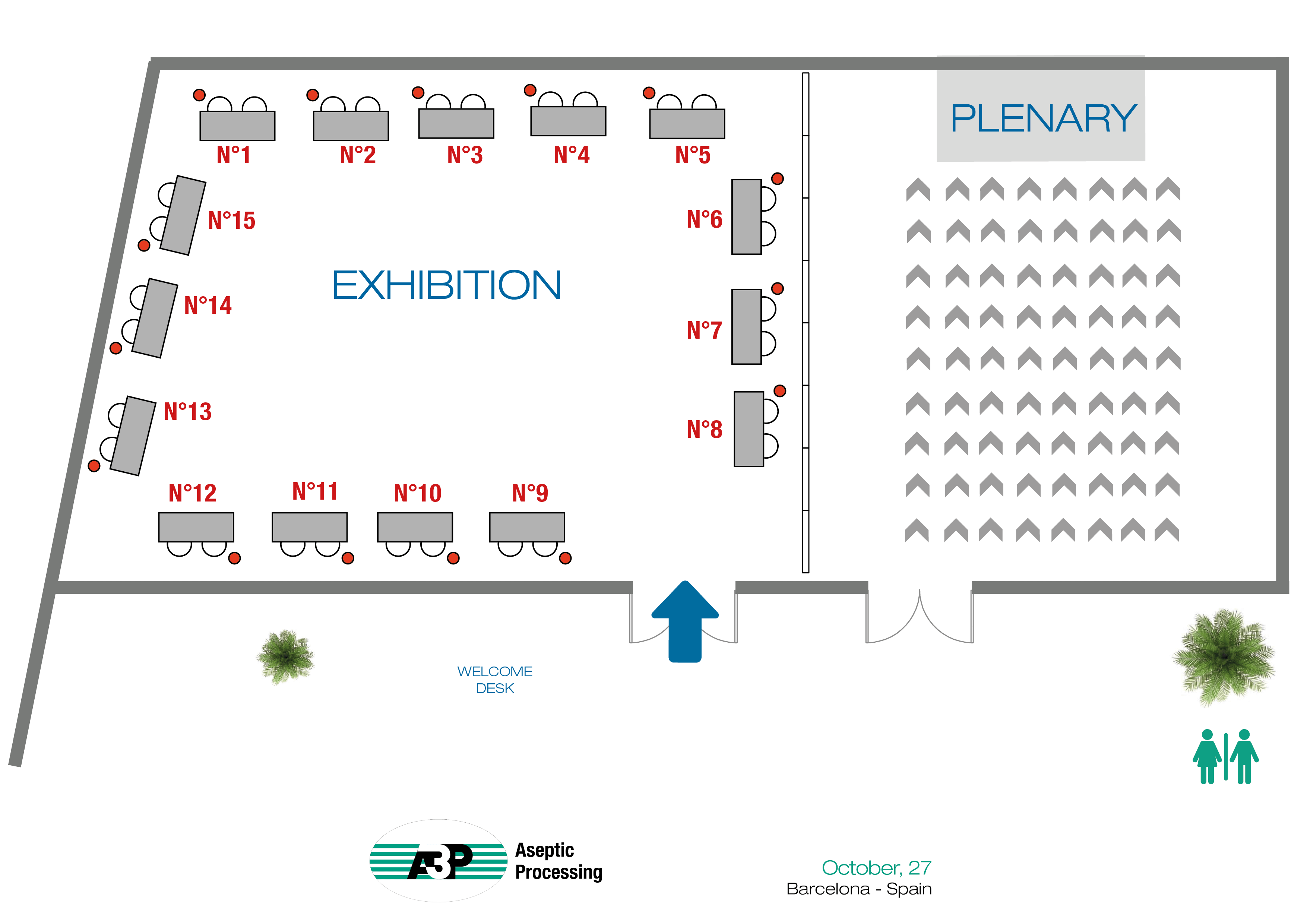
| Company | Table Top | Company | Table Top | |
| FPS PHARMA | 1 | SALAMANDER U | 9 | |
| DEVEA | 2 | MK-VERSUCHSANLAGEN UND LABORBEDARF e.K | 10 | |
| MESALABS | 3 | OPTIMA | 11 | |
| STERIS | 4 | BIOMERIEUX | 12 | |
| LITEK PHARMA | 5 | JACOMEX | 13 | |
| MARCHESINI | 6 | BECTON DICKINSON | 14 | |
| ECOLAB | 7 | JCE BIOTECHNOLOGY | 15 | |
| TISELAB | 8 |
Services included with the Table Top stand
- A dedicated exhibition area where you can install your products and communication tools: umbrella stand, roll-up, display, product, …
A basic equipment including a 180×80 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker - Breaks and meals as indicated in the program
- Illimited Wifi
- A badge that gives you free access to the entire technical and scientific programme.
- The list of participants to the event
Contact
Depending on your requirements and preferences, you can contact Florian Canuto:
Phone number: +33 (0)7 61 55 24 36
Email : fcanuto@a3pservices.com
How to access
Address
Plaça de Llevant, s/n, 08019 Barcelona, Spain
> Metro : El Maresme Fòrum
> Tram : Fòrum

