Summary
- Limit between GMP Part I/ Part II : Applicability to biological products
- Moving One Unit Operation At a Time Toward Continuous Biomanufacturing
- « Close Collaboration Maximizes Value of Engineered Solutions and Saves Time in Start-Up »
- Improving Single Use Bioreactor Design and Process Development. New Research Towards Intensifying Seed- Train & Scale-up Methods Using 5:1 Turn- Down
- Qualification approach for the validation of real-word shipping in single-use systems
- Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in BioBLU 0.3c Single-Use Bioreactors
- Single Use & Stainless Steel: complementarity or fight?
- Low Endotoxin Recovery (LER) is today one of authorities serious concerns regarding pyrogen testing
The complexity of biopharmaceutical manufacturing processes requires continuous improvement. As shown in figure 1, the expansion of manufacturing capacity worldwide has resulted in the multiplication of links between production facilities as well as the increasing need for storage or transportation of media, intermediate, BDS, and drug products between sites over the world.
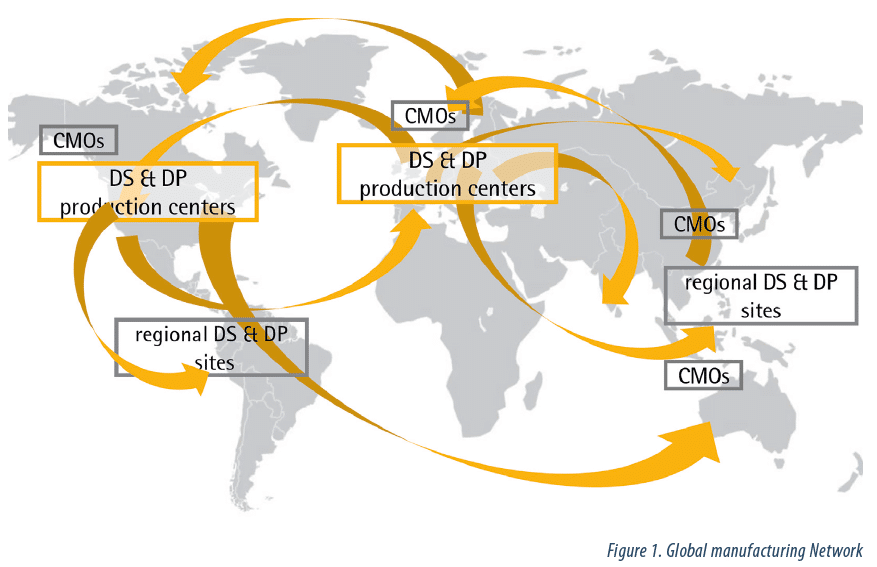
Outsourcing to contract manufacturing organizations (CMOs) offers a solution to the capacity constraints and reduces the total risks associated with building internal capacity; however, a robust and validated manufacturing process (2), including product transportation between facilities, is then required.
Single-use technology (SUT) continues to expand because of its potential for reducing both capital and operating expenses. The growing adoption of single-use, especially in critical process steps, has increased the need for product quality, robustness, and integrity. Depending on the manufacturing process organization and the level of outsourcing, the challenge of safe and robust BDS transportation becomes a crucial step from a risk analysis point of view. These shipments, increasingly performed in single-use bags, require thorough validation protocols to demonstrate their integrity and protection of the drug substances. The ASTM (3) and ISTA (4) both provide standardized validation methods using a variety of simulated shipping hazards. These tests are qualitative by nature with simple pass-fail criteria, therefore it is important that the robustness and failure modes of shipping systems are evaluated under real transfer conditions.
Regulatory background
Regulatory agencies like FDA (5), EMA (6) or EU (7) emphasize the need for end-user to ensure that their drug processes produce consistent and reproducible results which meet the quality standard of the drug product. Validation is “Establishing documented evidence that provides a high degree of assurance that a specific process” including shipping “will consistently produce a product meeting its pre-determined specifications and quality attributes” (FDA).
A properly designed system will provide a high degree of assurance that every process step, including shipping has been properly evaluated before its implementation.
In the biopharmaceutical industry, qualification and validation are intended to demonstrate that the manufacturing process provides the desired level of compliance of the product and specifically its activity, sterility and potency. Qualification of a shipping system and equipment is part of the process validation.
According to the PDA technical report N°66(8), “Shipping systems must be qualified for their intended use through proper design and testing in consultation with a packaging engineer. The transportation routes must be defined for international shipment. A risk assessment for vibration, handling, delays and seasonal variation should be established”.
International standards description
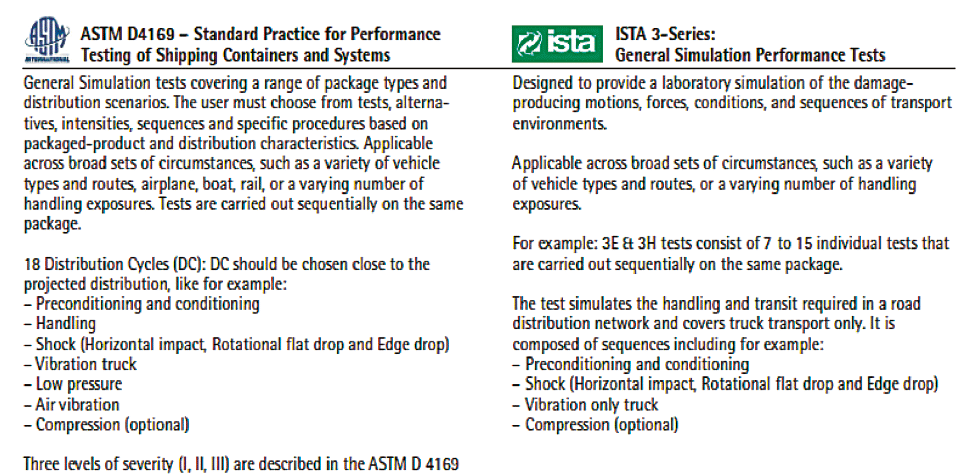
ASTM or ISTA are well-known standards for shipping systems. They are aimed to compare or evaluate the effectiveness of protective packaging and / or a packaged-product’s ability to withstand the hazards of distribution. Table 1 briefly describes the main features of ASTM and ISTA standards.
The level of severity must be defined according to real shipment condition in addition of desired safety margin. There is no official ASTM claim and suppliers can only claim that they pass ASTM selected tests described by the standard. Therefore, knowing the distribution cycle, schedule, duration, severity level, and acceptance criteria are mandatory to understand the validation performed on the system.
Moreover, the suitability with the intended use can only be proven by end-users; these conditions might differ from one site to another or from one product to another.
The definition of the system to be tested and the testing program need then to be carefully assessed and justified. The selection of the right testing parameters and the tested samples’ configurations can be done only by knowing the transportation cycles and the type of impact perceived by the load during the transportation, thus requiring preliminary testing and analysis.
Qualification approach
The supply of process solutions in large-volume bags, from point of manufacture to point of use is a well-established practice that involves the following elements:
• A bag designed to fit a rigid wall outer container
• A rigid wall outer container such as a plastic drum or tote or a stainless-steel bin
• Secondary packaging materials (e.g., dunnage) and lids or other mechanical devices to suppress the fluid wave action in the bioprocess bag.
Transportation of process solution in small-volume bags is also a common process that requires less complex packaging solution. The exception is the transportation of frozen materials that necessitates temperature-resistant materials and cold-chain logistics.
The PDA TR 66 has highlighted specific factors of importance for transportation that must be considered by end-user. These factors are:
• Dimensional factor (i.e., volume to be shipped and dimensions of the shipper)
• Mode of transportation, whether it’s ground, air, rail, boat, or combination of more than one mode of transportation. Metrics must include hold time on tarmac
• The associated environmental conditions (temperature, humidity, pressure, and variation)
• Functionality (i.e., forklift access, stack ability of outer container, access to fill and drain port, secondary container to collect leak)
• Room classification such as fill and drain procedure to maintain sterility • Logistics (e.g., external shipper, cold-chain logistics).
SUT shipping system composed of a bag and a stainless-steel bin should ensure safe shipment (i.e., no loss of integrity and no loss of product sterility). It can be granted by the mechanical robustness of the shipper. The objective is to verify that no leaks occur during transportation.
Qualification and validation of the shipped product can be performed in real shipment with monitoring or in simulated shipment according to ASTM D4169 or ISTA.

The protocol of test needs to correlate to the projected life cycle phase of the shipped unit. As a first step, knowledge of shipped product and the type of transportation (mean and sequences) are key to understand the shipping cycle and provide the adequate safety margin during qualification testing program.
A typical distribution sequence between two plants in the biotech industry is described in figure 2.
The choice of the testing program needs to fit the type of tested load and the parameters needs to be determined with a rational based on the shipping conditions and/or experience and packaging expertise in order to understand the impacts by transportation phases.
The next step is to define the severity of testing (level and duration). The level of severity should been defined according to a desired safety margin chosen over the real shipping tests impacts. The combination between real shipping tests and laboratory tests for the system characterization with ASTM D4169 and ISTA3E provides the relevant data helping to select the appropriate qualification method, parameters and acceptance level to qualify the shipping system (bags + shipper).
Conclusion
Shipping is indeed complex and the user should not be assuaged simply by vendors’ claims about regulations (i.e., claims of being “ISTA certified” or “ASTM compliant”). It is important to also understand what is behind each claim and verify that it is applicable to the product’s intended use. The end-user should understand the trial conditions used in the vendor tests and compare them to its application. The acceptance criteria (bag and shipper), the protocol, and trial conditions shall be discussed.
Shipping validation needs to be carefully defined in close collaboration between end-user and vendor, with parameter setting linked to actual use. Collecting vibration data on the real use will help the end user and the vendor to understand the physical constraints of the shipping mode and select the best protocol to replicate them in laboratory testing. The limits of the system should be defined with knowledge of the safety margin and be tested under real packaging and real transport conditions.

Katell MIGNOT – SARTORIUS STEDIM BIOTECH
katell.mignot@sartorious.com
Share article
References
[1] N. Riesen, R. Eibl, “Single Use Bag Systems for Storage, Transportation, Freezing, and Thawing” in Single-Use Technology in Biopharmaceutical Manufacture, Dieter Eibl and Regine Eibl Eds. (John Willey & Son, Hoboken, NJ, 2011.
[2] S.D. Jones and H. Levine 2005, BioExecutive International 3, 2–5, 2005.
[3] ASTM (American Society for Testing Materials) D4169: Standard Practice for Performance testing of Shipping Containers and Systems, 2014
[4] ISTA (International Safe Transit Association): General Simulation Performance tests
[5] FDA, Guidance for Industry Process Validation: General Principles and Practices, January, 2011.
[6] EMA,Guideline on Process Validation for the Manufacture of Biotechnology derived Active Substances and Data to be provided in the Regulatory Submission, April 2014.
[7] EU, European Commision, EU guidelines for Good Manufactruing Practice (Brussels, February 2014).
[8] PDA, Technical Report N°66, “Application of Single-Use Systems to Pharmaceutical Manufacturing”, 2014.




