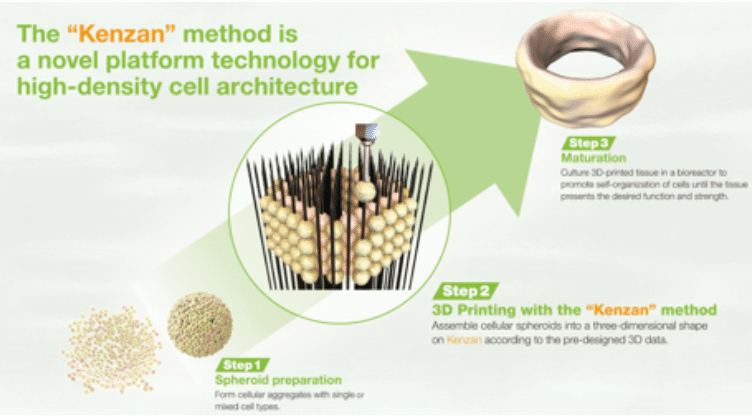
The techniques available for the microbiological monitoring of products and manufacturing environments have come a long way in the last few decades. Even though enumerating microbial cells by counting colonies on an agar filled Petri dish is still a common technique, a variety of more sophisticated and rapid methods are making their way into the quality control microbiology laboratory. These Rapid Microbiological Methods (RMM) exploit chemical and physical methods developed to elucidate the structure of cell components and the functions of biomolecules. These methods are then applied to the detection, enumeration, and identification of microorganisms that may be present in pharmaceutical, food and beverage, water and other samples submitted to the quality control labs. Thus, the simple view of a bacterial cell as a tiny living entity so small as to be invisible to the naked eye, and countable only by either microscopy or culture, is changing to a much more complex picture encompassing the most intimate details of the cell structure and the biomolecules that form it.