Sommaire
- Validation Strategy of Viral Decontamination Methods, a quick overview
- Antibody-Drug conjugate Manufacturing Techniques
- Robust and Convenient Single-use Processing
- Cahier Pratique – Quality by design applied to viral safety of Biologicals: Case studies & workshop discussion summary
- Mass spectrometry as a powerful tool for the characterisation of monoclonal antibodies in the context of comparability studies
- Chromatographie Continue : Solution d’amélioration des performances de procédés et « debottlenecking » des capacités de Bioproduction
- Protein A Affinity Chromatography for Efficient Fab Purification
- Enabling Higher Post Protein A Product Purity Using Novel Chromatographic Clarification Approach
Antibody based therapeutics account for 40% of the entire biotech drug market. In 2012 alone, the total antibody drug production exceeded $50 Billion in sales value (1). Monoclonal antibodies are becoming the leading driver of the therapeutic product pipelines. Together with the increased number of drug candidates comes the challenge of ensuring that an adequate, low cost, safe, and scalable manufacturing capacity exists and is able to address the ever increasing complexity of these bio-therapeutics.
While a lot of advances and optimizations have been made to the classic antibody production process to make it scalable, reproducible, and commercially viable, the basic structure of the process has changed little and significant challenges remain. Increasing cell densities, longer cell-culture duration, and higher product titers have challenged the basic design of the downstream train to deal with additional cell mass, cell debris, host cell protein, DNA, adventitious and endogenous viruses, and other impurities.(2)
At the front of the purification train is the clarification stage that clears the cells, cell debris, and the other insoluble aggregates. Typically, a combination of the centrifugation, depth filtration, and microfiltration is deployed to reduce the stream turbidity from over 1000 Nephelometric Turbidity Units (NTU) to under 10 NTU (3). The process stream containing the product is then loaded onto the Protein A column which acts as the primary capture step (4). Protein A, in turn, takes on the brunt of the work as it is exposed to host cell contaminants, including proteolytic enzymes, a large concentration of impurities that non-specifically bind to the column side by side with the mAb product, and denaturing cleaning conditions after every cycle (5).
As the result, the Protein A capture step is stressed to live up to its promise of purity and robustness, forcing a number of additional steps to be implemented downstream of Protein A.
The recent focus on chromatin and chromatin related impurities (6, 7) only further highlights the importance of developing a strategy for targeting and removal of the bulk of soluble contaminants upstream of the Protein A column.
Until recently, efforts to design an effective clarification train that will protect the Protein A column not only from macroscopic debris, but also from high concentrations of soluble DNA and associate HCP contaminants have been complicated by the inability to combine depth filter clarification properties, ion exchange chromatographic adsorption, and bio-burden reduction of a microscopic filter in a robust and cost-effective manner. (8) Traditionally, such implementations would include a depth filter followed by a membrane ion exchange flow-through device, followed by a 0.2 um filter. This, however, is more expensive, adds additional steps, and decreases process robustness.
Recently, Angelines Castro-Forerro et. al (9). demonstrated such a strategy using a 3M Emphaze™ AEX Hybrid Purifier – a functional non-woven based anion-exchange clarifier. This product uses a highly charged Q-functional non-woven polymer matrix (a hydrogel) to clarify the cell culture fluid (CCF) stream as well as to effectively remove DNA ahead of Protein A. The result was > 10 fold reduction of HCP and DNA in the Protein A elution pool. In addition a dramatic decrease in the amount of hard-to-remove HCP and DNA contaminants left on Protein A resin after product elution was observed. If such a strategy can be implemented effectively in the industrial process, it will greatly enhance, not only the affinity capture step efficiency and cycle lifetime, but also has the potential to simplify the polishing train downstream of the capture step (6).
Herein we describe an implementation of such a strategy by incorporating chromatographic clarification through the use of the Emphaze™ AEX Hybrid Purifier in an industrially relevant process. The clarification performance is assessed, as well as its effect on post Protein A HCP and DNA contamination.
Experimental
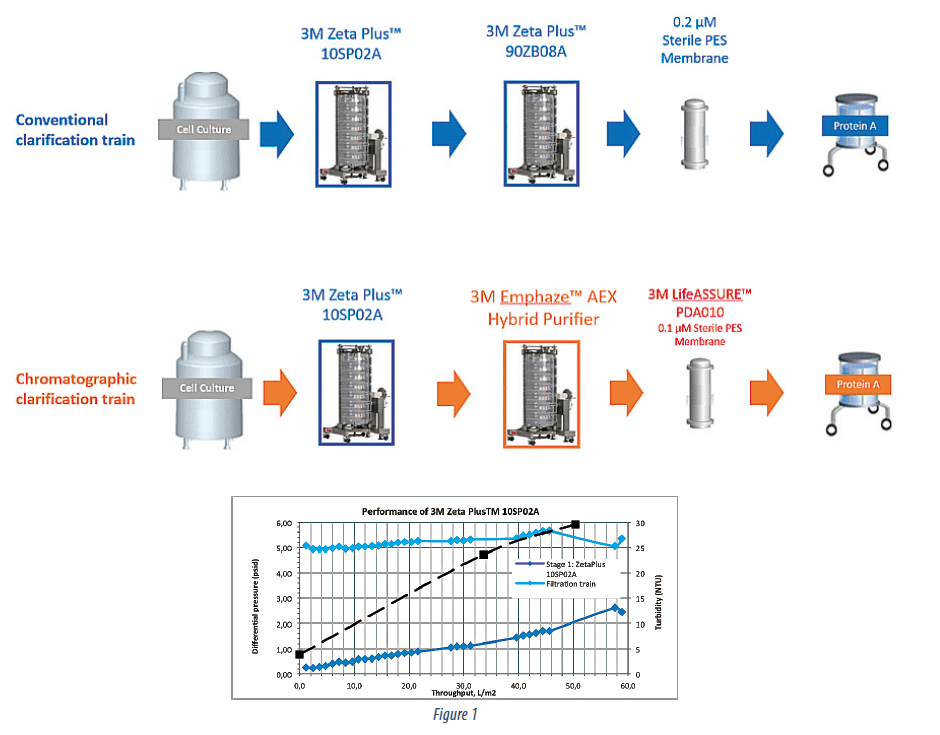
The standard CHO cell culture (cell density of 2.8*106 cells/ cc, viability of 52 %) expressing a recombinant mAb product (IgG1, pI = 7.8) was clarified. Depth filtration clarification process, incorporating, either 3M Zeta Plus™ 60SP02A in a single stage, or 3M Zeta Plus™ 10SP02A follows by a 3M Zeta Plus™ 90ZB08A was replaced with a dual stage approach utilizing 3M Zeta Plus™ 10SP02A as the primary cell removal stage followed by the 3M Emphaze™ AEX Hybrid Purifier as the second stage in a 2:1 area ratio respectively (Figure 1).
We also replaced the standard 0.2 μm filter with a 0.1 μm (3M LifeASSURE PDA010) sterile filter. Since the Emphaze™ AEX Hybrid Purifier reduces the DNA down to very low concentrations and contains a 0.2 μm qualifying zone, it offers excellent protection for a 0.1 μm sterilizing membrane filter.
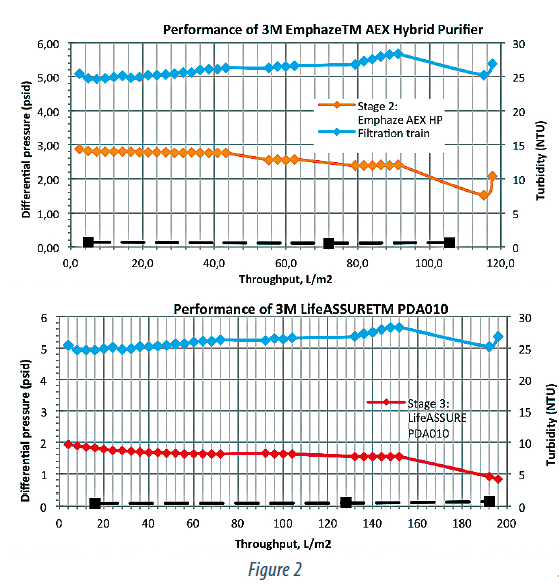
Figure 2 summarizes pressure and turbidity data across the 3 stages of this train (Zeta Plus™, Emphaze™, and LifeASSURE™). The Zeta Plus™ stage behaves in a typical depth filtration manner with linear increase in turbidity and pressure throughout its lifetime. Unlike the Zeta Plus™ however, the AEX Hybrid Purifier offers constant outlet turbidity and pressure.
This is consistent with the fact that the chromatographic clarification relies on capture by charge of the hydrogel rather than by the tortuous path traversal. Thus one would expect that the pressure would remain constant until the charge capacity of the hydrogel is exhausted. This type of approach provides excellent control of and stability during the clarification process.
The pressure across the 0.1 μm stage is also low and constant indicating that AEX Hybrid Purifier provides excellent protection of the sterilizing membrane filter stage. It was previously reported (9) that the AEX Hybrid Purifier removes DNA-protein complexes having hydrodynamic sizes in the range of 100-200 nm and the size of the particles coming out the chromatographic clarification stage was limited to ~ 30-40 nm in that study (IgG1 hydrodynamic radius is 11-12 nm).
Together with the pressure data obtained in our experiments, this would suggest that even a 0.1 μm filter will have, virtually, unlimited throughput and can be sized based on flowrate alone. This is, clearly, an advantage at manufacturing scale as the typical sterilizing filter stage post clarification is designed based on throughput.
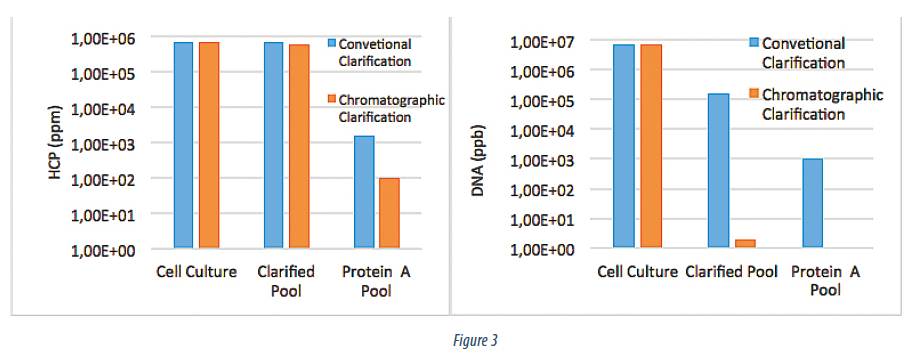
The Clarified Cell Culture Fluid (CCCF) obtained using the chromatographic clarification train was purified on Protein A column (MabSelect, GE) using a standard purification protocol. Figure 3 summarizes HCP (performed via Cygnus CHO HCP kit) and DNA (performed via Q-PCR) at the preclarification stage, post clarification stage, and in the Protein A elution pool. What is clearly evident from the data is the high degree of the DNA removal by the Emphaze ™ AEX Hybrid Purifier and very high mAb product purity post Protein A purification. The DNA is cleared to 2 ppb (> 6 LRV), that is at the limit of detection for the Q-PCR. High degree of removal of the interfering DNA contaminant enables qualitatively higher purity in the Protein A elution pool at 1 ppb DNA and 100 ppm HCP. This is the case even when the HCP reduction over the entire clarification train is only ~ 20 %. This is > 10 X improvement in post capture purity compared to the conventional approach and is a step change in the performance of the Protein A step and, thus, the purification process itself.
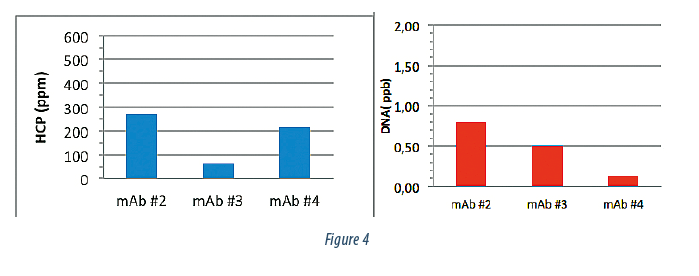
We then purified 3 additional mAb products using the same chromatographic clarification strategy to understand the consistency of this approach. Figure 4 summarizes the post Protein A purity data. All 3 products were eluted off the Protein A with very high purity at, or near, the quantifiable limit in terms of DNA and HCP content.
Discussion
Dramatic and consistent increase in product purity post capture to the near injectable level brings into view a number of process development paradigms that are critical to the development of the mAb purification processes of tomorrow.
The first is platformability of the mAB purification process. The main premise and driver of rapid bio-pharmaceutical drug development over the last 10 years has been the principle of platforming of the key steps in the discovery-to-clinic-to-manufacturing process.
Antibody scaffold only further facilitated this through a unified bio-pharmaceutical product architecture. Yet, purification process remains, largely, a custom-designed approach for each mAb product, primarily due to inconsistent and relatively low post Protein A purity, which dictates the engineering of the polishing train.
Chromatographic clarification approach solves this problem getting one step closer to building a unified polishing train for every product. Whether this unified polishing train strategy is one process configuration or a rapidly selected set of several standard strategies is immaterial. The high and consistent purity of the product post capture enables a consistent and predictable approach to polishing, and thus, to the entire purification process. This, in-turn shrinks the process development time and accelerates the discovery-to-clinic-to-production pipeline.
The second critical element enabled by the high post-capture purity is a step-change in cost and productivity of polishing trains. The size, cost, and architecture of the polishing part of the mAb purification process is dictated by the amount and consistency of the impurities that are left in the process stream post Protein A. High product purity enables more compact and cheaper polishing trains. This element becomes more critical as the process scale rises and its complexity and cost grow non-linearly.
Since the chromatographic clarification enables more than an order of magnitude reduction in DNA and HCP product stream contaminants, one can reduce the size of the required process steps, such as the AEX polishing step, accordingly. In cases, delete comma where viral clearance requirements are abbreviated, such as for preclinical uses, the polishing train may be completely eliminated. At large scales this leads to a significant risk reduction and cost savings. More importantly it enables one to deploy more purification trains into existing facilities, delaying the often politically and financially challenging capital expenditures for construction of new manufacturing plants.
Conclusion
Efficiency of the product capture step has always driven the performance of the entire process. Increases in efficiency of the capture step in the mAb purification is a subject that has been relentlessly studied for the last 10+ years. Genomic DNA and associated chromatin complex has been identified as the likely major component interfering with the Protein A chromatography.
While methods, such as cell culture acidification and treatment, do exist to reduce the DNA in the clarified cell culture media, they have, either been limited to academic space, or have shown to be of limited use in the industrial space due to product stability and process control issues.
Here we demonstrate a novel alternative method of chromatographic clarification approach using an anion exchange clarifier 3M Emphaze™ AEX Hybrid Purifier to reduce the DNA down to near the limit of detection at clarification. The result of this DNA reduction produces a step change in the purity of the product eluted off the Protein A column. Unlike other strategies, this approach enables a high degree of DNA reduction in a scalable, flow-through, single use system that is industrially viable and rapidly deployable in a commercial process.
The consistently high purity of the product post capture streamlines development, lowers manufacturing costs and improves process efficiency that comes with high process platformability and robustness.

Alexei VOLOSHIN – 3M PURIFICATION INC
avoloshin@mmm.com

Dmitri SMIRNOV – 3M PURIFICATION INC

William WESSEL – CATALENT INC

Ian COLLINS – CATALENT INC

Steven HAGER – CATALENT INC
Partager l’article
Bibliographie
1. Therapeutic antibodies: Market considerations, disease targets and bioprocessing, International Journal of Pharmaceutics, John G. Elvina, Corresponding author contact information, E-mail the corresponding author, Ruairidh G. Coustonb, Christopher F. van der Walleb, Volume 440, Issue 1, 2 January 2013, Pages 83–98
2. New Biotechnology, Volume 28, Issue 5, September 2011, Pages 458–463 Advances in the production and downstream processing of antibodies
3. Pharmaceutical Technology MARCH 2003, The Clarification of Bioreactor Cell Cultures for Biopharmaceuticals, David Yavorsky, Robert Blanck,* Charles Lambalot, and Roger Brunkow
4. Purification Strategies to Process 5 g/L Titers of Monoclonal Antibodies, Mar 2, 2009 By: Melody Trexler-Schmidt, Stefanie Sze-Khoo, Amber R. Cothran, Binh Q. Thai, Sandy Sargis, Benedicte Lebreton, Brian Kelley, Gregory S. Blank , BioPharm International Supplements
5. Journal of Chromatography A, Volume 1216, Issue 31, 31 July 2009, Pages 5849-5855, A mechanistic study of Protein A chromatography resin lifetime, Canping Jianga, Jing Liub, Michael Rubachaa, Abhinav A. Shuklaa, Corresponding author contact information, E-mail the corresponding author
6. Emerging Challenges to Protein A Chromatin-Directed Clarification Enables New Purification Options , Pete Gagnon, Bioprocess International
7. 14 Gagnon P. Disruptive Methods for Purification of Biosimilar and Biobetter Proteins. Presentation, Biopharmaceutical Development and Production Conference, 25 February–1 March 2013, Huntington Beach, CA. Organizer: IBC (Westborough, MA).
8. Recovery and purification process development for monoclonal antibody production, mAbs 2:5, 480-499; September/October 2010; © 2010 Landes Bioscience.
9. Anion-Exchange Chromatographic Clarification: Bringing
Simplification, Robustness, and Savings to MAb Purification, BioProcess International, June 2015.