Sommaire
- Annex1 & CCS → How to leverage mathematics & IT tools for driving improvement?
- CCS → Part 1. Deep dive on CCS understanding
- No detail left behind: A holistic approach to opening authorization in Sterile Manufacturing
- Production of incretin mimetics → Weight under control
- The next generation of reagents for Endotoxin Testing for the next generation of scientists in the pharmaceutical / medical device industry
- Le secret des produits pharmaceutiques et cosmétiques réussis, de la formulation à l'application
- A Toolbox for an Effective Technology Transfer
- Excess heat in pharmaceutical industry
Annex1 & CCS → How to leverage mathematics & IT tools for driving improvement?
The latest update to Annex 1 of the EU GMP guidelines marks a major shift in sterile medicinal product manufacturing. It moves away from strict compliance towards a more integrated, science and risk-based approach. A key addition is the requirement for a Contamination Control Strategy (CCS), which unifies all contamination control efforts into one documented framework. The CCS is now a core element of quality assurance. It requires manufacturers to thoroughly assess all potential contamination sources and demonstrate a state of control through facility design, equipment, personnel behavior, monitoring, and more… The revised Annex 1 also introduces lifecycle thinking, treating contamination control as a continuous, evolving process

This shift raises expectations for manufacturers, demanding more transparency, traceability, and accountability. Annex 1 is now both a regulatory guide and a strategic tool, promoting collaboration and stronger risk awareness. At its center, the CCS ensures contamination risks are not only identified but actively controlled, supporting a culture of proactive quality for product safety
Gap versus Risk, what is the difference?
Gap assessment is a structured method used to identify the difference between a current state and a defined standard, goal, or requirement. It helps evaluating how well existing practices or systems align with expectations like regulations or quality standards. The “gap” is the shortfall between what exists and what is needed, and identifying it leads to corrective actions and ongoing improvement. The aim is not to assign blame but to clarify what is lacking and direct efforts where they are most needed.
On the other hand, Risk management is a decision-making process focused on identifying, understanding, and addressing uncertainty. It involves recognizing factors that may impact an organization’s ability to meet its goals, assessing the likelihood and impact of these risks, and deciding how to respond. This may mean lowering the chance of the risk, limiting its impact, or accepting it if it’s minor. The goal isn’t to remove all risks (an unrealistic aim) but to make them visible and manageable.
Application to Annex 1 and the CCS
On the other hand, Risk management is a decision-making process focused on identifying, understanding, and addressing uncertainty. It involves recognizing factors that may impact an organization’s ability to meet its goals, assessing the likelihood and impact of these risks, and deciding how to respond. This may mean lowering the chance of the risk, limiting its impact, or accepting it if it’s minor. The goal isn’t to remove all risks (an unrealistic aim) but to make them visible and manageable.
By moving logically from compliance needs to risks identification and continuous improvement, the CCS provides regulators and internal stakeholders with clear evidence that contamination risks are understood, controlled and actively reduced over time.
The main drivers for the establishment of the site’s Contamination control Strategy are in relation with the design, execution, and monitoring. The strategy is built on a foundation of essential principles and practices, each contributing to a comprehensive approach to ensuring product safety and quality. Key elements as described below basically give the skeleton of the Strategy.
BASICS
The contamination control strategy is grounded in Good Manufacturing Practices (GMP), quality risk management (QRM), and a culture of scientific awareness and procedural discipline. Employees are trained to see GMP not just as rules, but as essential to their daily responsibilities.
CONTROL
The strategy emphasizes preventing contamination through smart facility design, proper flows of people and materials, and strong cleaning and disinfection routines. All operations are structured to support cleanliness, with validated checks and proper waste handling to avoid contamination.
VALIDATION
Validation ensures all controls are effective. Rooms are qualified, cleaning and sterilization methods are tested, and aseptic process simulations confirm the system can reliably produce sterile products. Any issues trigger swift, risk-based actions.
MONITORING
Monitoring plays a central role. Environmental conditions, utilities, and products are regularly checked. This data helps detect trends and supports improvement. The system evolves through ongoing review and prioritization of risks.
GOVERNANCE
It keeps the strategy strong. Clear policies and procedures are maintained, compliance is monitored, and problems are resolved through defined escalation processes, ensuring a consistent and accountable contamination control approach.
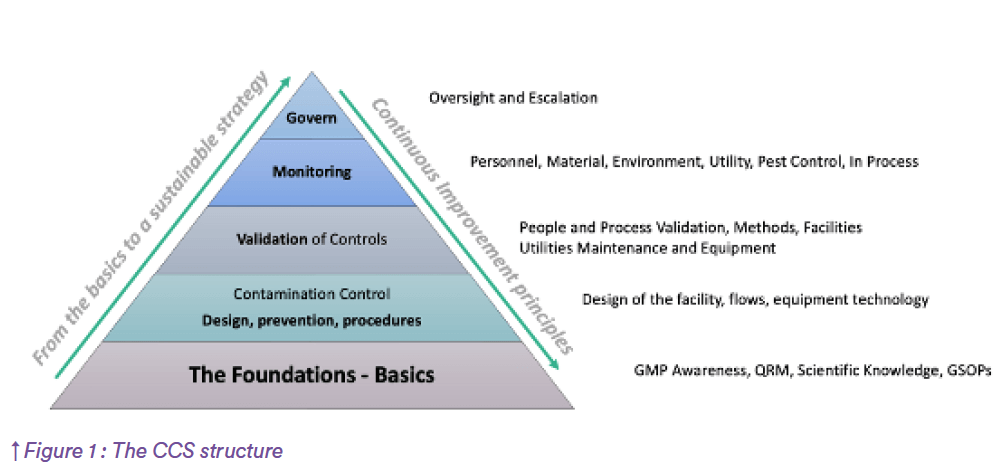
Integrating elements and building a Risk Based Strategy
The Contamination control Strategy elaboration is a staged approach starting with Gap assessment and includes of
course KPIs. The below model describes the entirety of the strategy elaboration, from the design to the monitoring Once the Gaps have been identified and categorized from Compliance and Business perspective, the action plan must be rolled out, and in parallel the strategy defined for a sustainable sterility assurance. This is achieved through the use of Risk assessments, standards implementation, etc.
The strategy will be documented in a so called “CCS document” as per Annex one requirement, that aims to be easy to navigate and provide all necessary information supporting the contamination control strategy. The CCS structure may vary depending on the process, or the complexity, but will always provide the elements as described in figure 1. The strategy for contamination control must be risk assessed to ensure its effectiveness. This will include criteria such as its added value on patient safety, its robustness from a business perspective, but must also include the complexity of the strategy, and the oversight from the Quality Organization. The CCS itself is subject to continuous improvement based on risks.
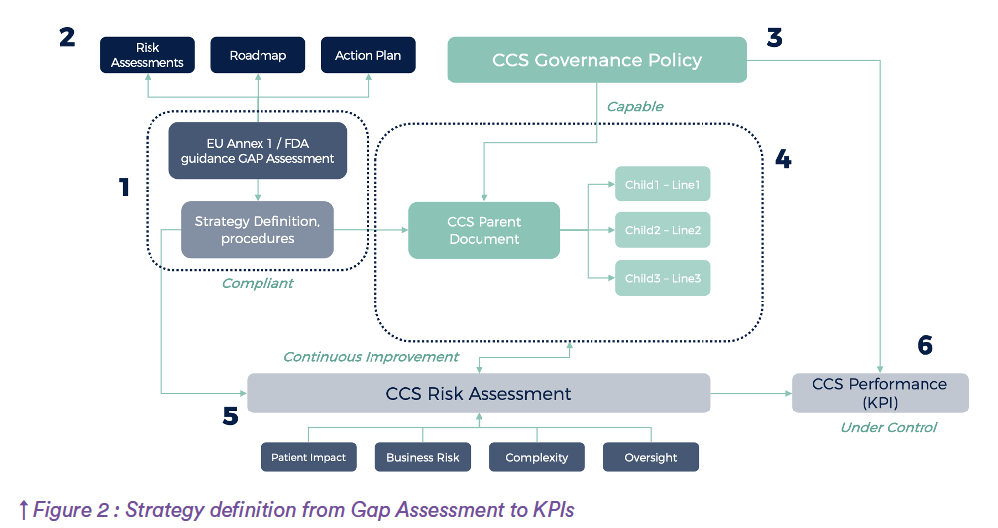
A Gap Assessment, yes! But let’s think different.
When performing a Gap assessment regarding compliance to Annex 1, several criteria may be used. The goal should not only be to respond to the question “Gap” or “no Gap”, it’s never as simple as that, but rather to provide at the same time an information regarding the Risk related to the GAP. That’s explained later in this article, but let’s talk a little bit about mathematics first.
Why thinking in ratios is better than considering differences?
When we compare two positive quantities, or values, we can look at their difference (subtract one from the other) or their ratio (how many times one fits into the other). The second approach gives a quicker and actionable information specifically when dealing with lots of Data.
How our minds reinforce this
Experiments in psychology show that people detect change in proportion to the relative difference, not the absolute one. The tiniest change you can notice in a stimulus is roughly a fixed fraction of its current level. In other words, human perception is tuned to ratios. That said, we can imagine that a Risk Assessment using Risk Priority Numbers can easily be compared to the maximum score possible, thus telling us how big the Risk is compared to the worst case situation of a 100% Risk. Interesting isn’t it? But that’s not all.
Why a logarithmic scale helps
By writing each value as its logarithm (log a, log b). The distance between two points on a log axis is simply log (a ÷ b), the logarithm of the ratio. This single step gives several benefits:
• Equal spacing for equal ratios. Doubling, tripling, or halving always occupies the same visual width, so tiny numbers are not squashed and huge numbers are not stretched.
• Multiplication becomes addition. Logarithms turn products into sums, which are easier to plot, fit with straight lines, and analyze statistically. Stable variability. Data whose scatter is naturally multiplicative (e.g., growth rates) shows a constant spread after logging, making standard tools such as least-squares more reliable.
• Unit-free comparison. The log. distance is unchanged if every measurement is rescaled, preserving objectivity.
What do we conclude out of it?
Ratios capture the true “relative size” of things, our brains naturally track change this way, and a logarithmic scale lays those ratios out in straight, even steps. That makes multiplication-based thinking, expressed through logs, the clearest and most efficient framework for comparing quantities at any scale.
Using a logarithmic calculation system, values are discriminated in the sense that lower values become less visible and higher values gain visibility.This enables the use of the full range of measurements and helps to better discriminate Risks for an accurate prioritization.
For the calculation, we can use a Log10 referential and divide the result of the calculation by the maximum achievable score, to obtain a result ranging from 0 to 100%.
The graph (Figure 2) beside is generated from random data to calculate a RPN (Risk Priority Numbers) using 4 risk factors (f1 to f4) ranked from 1 to 5 using the formula described, and shows this differentiation between logarithmic ratio (green) and usual linear models (red).
The way data are plotted on the chart enables a better discrimination and subsequently a far more efficient decision making process when it’s about setting priorities. Last but not least that doesn’t affect the comparative positioning of each item. A low Risks keeps low, and a high risk keeps high, but better separated. Having GAPs visualized in such a way permits the elaboration of a structured action plan. Let’s apply this
to Annex 1.
The Annex 1 Gap assessment
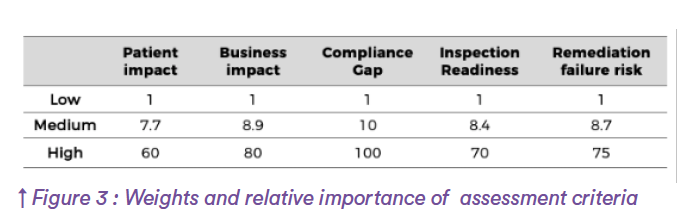
The risk scoring system used integrates five key factors: patient impact, business impact, compliance gap, inspection readiness, and likelihood of remediation failure. These are not weighed equally (gap severity carries the highest influence, followed by remediation effort, probability of failure, inspection preparedness, and patient safety impact.
Each factor is rated on a logarithmic scale (low, medium, high) and converted into weighted numerical values, allowing a Risk Priority Number (RPN) to be calculated for each compliance item.
Company strategy
Should it be for a launch of the facility or a routine re-assessment, criteria will have a different weight. The example below shows the Gap (including all of these aspect for each requirement) will be very precisely evaluated in alignment with the context of the company.
Values are indicative.
The intermediate value (Medium) corresponds to the half of the logarithmic scale between low and max values.
Gaps calculation
For each Gap, the calculation is made by multiplying the values for all of the 5 criteria, and dividing the log of the result by the log of the maximum values multiplied. It gives for each item of the standard a so called “success score”, not only based on Compliance (Gap/No Gap) but integrating all aspects in terms of risks. In parallel, the calculation is made regarding pure Quality and compliance risks (using patient and compliance criteria), and business risks (using Business and Remediation failure risk).Back to Annex 1, the compilation of all of these data provide a risk score representing all gaps for on given chapter (Indicative values):
When adding the Preparedness criteria (Inspection readiness), the areas of focus might be slightly different, and calculation (using ratio and logarithmic scale) enable an immediate visualization of areas of focus.
The outcome of this methodology is a multi-dimensional risk map, capturing both quality-related and business-related areas of focus. These are used to build a focus matrix, visually distinguishing critical issues that combine high compliance risk and high remediation difficulty. The most urgent priorities emerge where compliance risk and difficulty intersect. Each plot of the map represents a requirement from annex 1.
To translate risk scores into action, a prioritization system is applied. This assigns a four part code to each gap, derived from its overall success score and sub-scores in business urgency, preparedness, and failure risk. The resulting roadmap ensures that the most critical issues (those with high impact, difficult mitigation, and no solution) are addressed first.
These charts are being generated using Power BI capabilities based on calculation Data sets. enabling a possible periodic re-assessment using the tools, with updates sets of Data, after changes in the process, or regulatory updates for example.
This methodology goes beyond checklist compliance. It enables rational resource allocation, data-driven decision-making, and transparent communication. In doing so, it embodies the risk- and science-based mindset at the heart of Annex 1, where the aim is not only to meet standards, but to sustain a robust, justifiable state of control throughout the product lifecycle.
And what about KPIs?
In the evolving landscape of contamination control, the ability to measure, interpret, and respond to performance indicators is no longer a complementary feature it is foundational. Within the framework of the Contamination Control Strategy (CCS), Key Performance Indicators (KPIs) are not simply dashboard metrics; they are the analytical backbone that transforms the CCS from static documentation into an operational, verifiable system of assurance.
The proposed process for an effective oversight consists in a multi-layered, data-driven approach. Each component of Annex 1, ranging from premises and utilities to quality control and environmental monitoring, is mapped against five strategic pillars: basics, controls, validation, monitoring, and governance. These pillars form the structure of the CCS, and the KPIs provide the continuous signals that show whether each part is functioning as intended. What emerges is a system where every requirement of Annex 1 is mirrored by at least one measurable indicator, ensuring that completeness, traceability, and trending capability are embedded from the outset.
Metrics are normalized on a 0–100% scale, turning qualitative assessments such as the adequacy of cleaning or personnel training, into quantitative, auditable values. These indicators feed a digital scorecard, visualized through a Power BI dashboard, that not only shows current status but also highlights deviations, risks, and missing data. By incorporating multiple timeframes—quarterly for operational metrics, rolling 12-month windows for seasonal or low-frequency events, and biennial checkpoints for governance activities—the system ensures both granularity and long-term oversight.
Beyond static reporting, the dashboard allows for slicing data by CCS element, department, facility, or even specific process lines. It empowers leadership and quality teams to analyze trends, benchmark performance, and initiate timely actions. For regulators, the KPI structure provides immediate confidence: they are presented not with anecdotal narratives, but with a transparent, continuously updated demonstration of aseptic control.
This KPI-centric model embodies the Annex 1 emphasis on lifecycle quality management. By making contamination risk measurable, comparable, and actionable, the CCS is no longer just a risk strategy, it becomes an engine for sustained GMP performance and inspection readiness. In this context, KPIs do not just inform: they govern.
Partager l’article





