Sommaire
- Du Capteur au Serveur pour valider, produire, piloter, libérer… Les enjeux de l’évolution de l’automatisation du procédé à son pilotage par ses données
- Profiter de l’extension de la capacité de production pour établir de nouveaux standards en Automatisation de Procédés
- Cahier Pratique – Évaluation des fournisseurs en SI (Systèmes Informatisés)
- Du design à la production Retour d’expérience du point de vue engineering (La Vague 50)
- Extension, Revamping et Mutualisation de Systèmes de Contrôle Informatisés
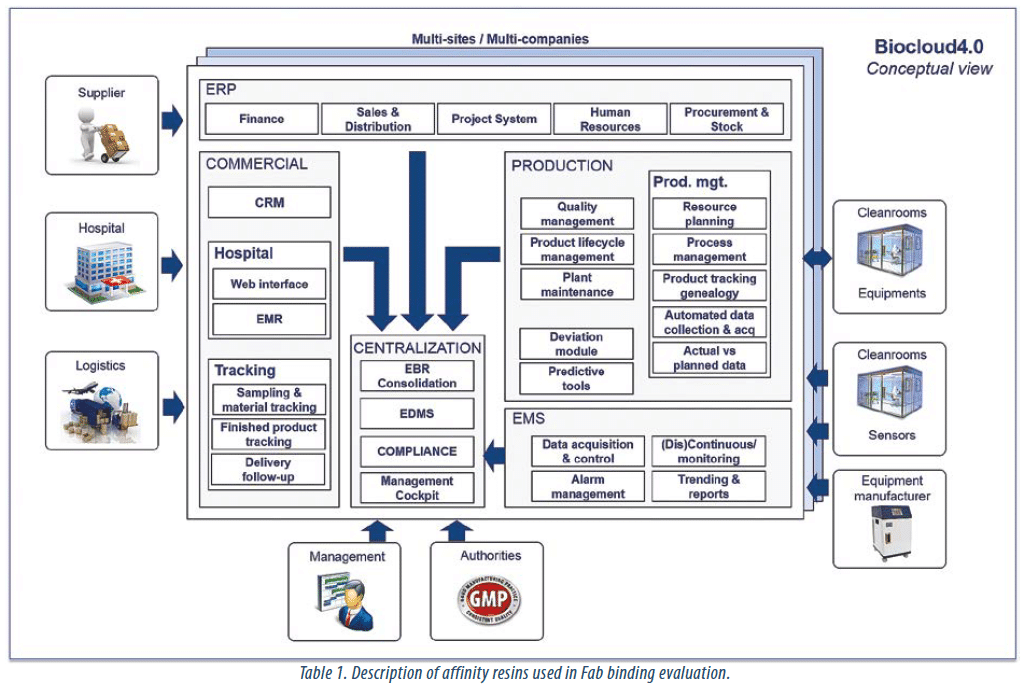
- BioCloud4.0 A patient-centric global IT solution of the new generation, designed for biotech organizations
- Difficultés du management des SI sur un site pharmaceutique
How an integrated IT solution will enable the biotech manufacturing units to drastically reduce the costs of producing advanced therapy medicinal products (ATMP). The health sector sees the emergence of new generations of innovative therapies, among which promising approaches are related to gene and cell therapies.

This evolution often leads to the deployment of patientcentric processes, implying not only dramatic changes in the bio-production processes but also significant increases of the treatment costs.
Specific needs of biotherapy companies
Contrarily to traditional therapeutic approaches for which companies of the pharmaceutical sector are structured for producing standard treatments (product-centric), some of the new biotherapy strategies are characterized by the individualization of the treatment production (patient-centric). As a result, traditional IT solutions (integrated management IT, productcentric) that are used by the companies of the pharmaceutical sector are no longer adapted to the needs of new generation biotechnological companies (patient-centric).
Those new companies also go through a structure and size transformation linked to their progression through clinical phases. They evolve from a “research lab” stage to the “industrial company” stage. Consequently, those companies are facing the complex challenge of creating the IT and technical infrastructure supporting the management, manufacturing and environment monitoring tools of their production sites. Ideally, the capacity of this infrastructure should scale to the evolution of the company activity volume. The transformation process is both difficult and risky. The adoption of a global, centralized, scalable solution verticalising all IT support functions will simplify that process, reducing risks and removing potential growth brakes.
More particularly, the specific needs of relatively young companies dealing with patient-centric production processes include the need to produce at an affordable cost, very flexible production rooms, the full integration of the treatment administration chain, and, last but not least, GxP1 compliant document management and qualification of the software solution.
Currently these companies have to deal with a series of limitations. The cleanrooms are highly wired, which reduces their flexibility and agility in adapting the production schedule. Sensors are rarely configurable remotely and do not allow for sufficient flexibility in data capture frequency variations, while management systems can only cope with a limited number of sensors. Traditional wireless communication technologies (Wi-Fi) generate largely inhomogeneous electromagnetic fields with a strong intensity in some areas and do not ensure a proper function guarantee (SLA) within a clean room (Faraday cage effect).
The limitations also apply to software solutions. Traditional GxP compliance solutions lead to unmanageable systems for autologous bio-producers, for example. Market-available ERP solutions are “product-centric” and not easily customizable (and probably would not be efficient) for supporting many asynchronous parallel patientcentric processes.
In order to address those major challenges, the biotech industry will need to adopt and deploy the principles of lean manufacturing and of the Industry 4.0 concept.
The proposition
Lean manufacturing and Industry 4.0 principles are the most promising approaches to address the above-described challenges: continuous supply chain management, connected machines, mass individualization, continuous follow-up and control, augmented operator, intelligent products… All these elements are likely to bring tremendous benefits to bio-manufacturing actors, as in other industrial sectors.
Internet of Things, wireless communication, Cloud technologies are the foundation technological building blocks that the Biocloud4.0 Consortium is going to adapt, extend, improve and integrate in order to build the BioCloud4.0 product.
BioCloud4.0 is an integrated system solution consisting of a new generation of hardware and software products (and associated services) enabling revolutionary bio-production methods, thanks to sensor miniaturization, a specific and reliable wireless technology, a technology integration box and patient-centric IT functions.
BioCloud4.0 aims at deploying the principles of lean manufacturing and Industry 4.0 within the production processes within small and large biotech and pharma actors facing patient-centric processes challenges. There is, anyhow, no doubt the whole pharmaceutical industry will also benefit from these developments.
The main development axes
• Create, develop and validate a new « Patient Centric » IT solution tuned to the specific requirements related to
bioproduction processes within clean rooms.
• Develop, optimize and validate a wireless radiating cable2 communication technology with high reliability, extremely low electromagnetic field intensity and wide broadband capacity.
• Develop and validate new intelligent, wireless, miniaturized, energy-autonomous sensor generations optimized to clean room conditions.
• Create and validate an integrated solution consolidating electronic and IT technologies (measurement, transmission,
processing, and management) for bio-production companies.
• Initiate a strong regional ecosystem of industrial and
academic partners for a long-term innovation strategy.
BioCloud4.0 research activities will be driven by the requirements of advanced-therapy medicinal products (based on genes, cells or tissues) production in clean rooms, as these companies combine the most technical challenges for the BioCloud4.0 solution.
The approach
The Sapristic group (ERP specialist and IT supplier for clean room environmental control through its daughter company BiiON) has led a strategic thought process which was extended in the framework of a working group coordinated by Agoria3 (with the participation of SEE Telecom and UCL – Université catholique de Louvain), and benefited from an Innofaster4 guidance proposed by the OpenHub of Louvain-la-Neuve.
Extensive discussions with target users have helped to clearly identify a market opportunity as well as the specific needs that are currently unsatisfied. This work has led to the strategic vision of what this project BioCloud4.0 implements: the development of a new integrated solution for information capture, transfer, processing and management structured around the patient (patient-centric), based on the principles of Industry 4.0, exploiting Cloud, IoT5 and innovative wireless technologies and suited to the specific needs of the new biotechnological companies.
To implement this strategic vision, the initial partnership has been extended to JUMO Automation and UCL for their expertise in industrial sensors, to ISSeP for their expertise in the analysis of electromagnetic fields and in particular the radiating cable6 technology and to UMONS (Université de Mons) for the network expertise.
The specific end-user viewpoint is represented by MaSTherCell a Contract Development and Manufacturing Organisation) and Novadip Biosciences (a biopharmaceutical company focused on new generation of autologous therapies from adipose stem cells adapted to hard and soft tissues reconstruction), target SME for BioCloud4.0, and complemented by the contribution of an external Advisory Board.
Expected deliverable: the BioCloud4.0 solution
The main project outcome consists of an integrated solution (here referred to as the BioCloud4.0 solution) for information capture, transfer, processing and management centered around the patient, based on the principles of Industry 4.0, and adapted to the specific needs of biotechnological companies dealing with patient-centric bio-production processes.
The BioCloud4.0 solution consists of the following modules:
• A new generation of geo-localized wireless sensors meeting the needs of the pharmaceutical sector.
• A wireless telecom solution suited for the health sector, based on the radiating cable technology, with high reliability, high throughput and low electromagnetic field.
• A cloud-based, integrated biotherapy-oriented, patientcentric Enterprise Resource Planning (ERP) solution supporting the whole production chain up to treatment delivery and administration including
√ A Customer Relationship Management (CRM) tool
√ A Quality Management System (QMS)
√ Modules for production management, Human Resources, Finance, Accounting, Supplier, Stock and Third-party management.
• A Manufacturing Operation Management System.
• An Environment Monitoring System (EMS); evolved from Mirrhia, which is currently commercialised.
• A centralization module (date base) allowing advanced reporting to management and authorities.
• The Mirrhia Box and an integration layer, allowing to easily connect all hardware and software components of BioCloud4.0.
The project BioCloud4.0 will stretch over 3 years. The project management strategy has been designed in an agile and evolutionary way in order to progressively integrate and validate the intermediary versions of BioCloud4.0 alongside the development. This allows making sure that validation operations can be done on partial (or “hybrid”) solutions during the project lifetime, so that these intermediary versions of BioCloud4.0 can go to market even before the project end.
Quality requirements for Life Sciences
To conclude, developing a software for the Life Sciences sector (especially the pharmaceutical sector) translates into specific quality constraints. These relate not only to the way the software has to behave but also how it has to be developed and tested. Respecting GAMP5 rules and V-shape quality framework are a must in this regard. A SaaS architecture will carry out other types of rules and constraints to comply with, rising the challenge at higher level of qualification.

Anne CASSART – BiiON
anne.cassart@biion.com

Pierre KAYENBERGH – BiiON
pierre.kayenbergh@biion.com
Partager l’article
Bibliographie
[1] GxP: Good (Anything…) Practice as a generic way to designate GMP (Good Manufacturing Practice) and any other types of Good Practices relevant for the addressed business.
[2] The radiating cable (or leaky cable, or leaky feeder) is a communication system, that presents significant advantages as compared to Wi-Fi technology (see https://en.wikipedia.org/wiki/Leaky_feeder).
[3] Agoria (http://www.agoria.be/) is the Belgian federation of the technology industry.
[4] See http://www.openhub.be/.
[5] IoT: Internet of Things.
[6] The radiating cable (or leaky cable, or leaky feeder) is a communication system, that presents significant advantages as compared to Wi-Fi technology (see https://en.wikipedia.org/wiki/Leaky_feeder). .