Sommaire
- L’intérêt de la désinfection automatisée des surfaces d’un RABS par decontamination aérienne
- Utilisation d’isolateurs individuels pour la thérapie cellulaire autologue
- Calculation of greenhouse gas (GHG) emissions expressed in CO2eq of an open system (AinB) compared to a closed system equipped with isolators (AinD)
- Exigences croisées de la norme ISO 13408-2 et de l’Annexe 1
- Employing Conductivity Measurements for On-site Residue Quantification
- LEAN. Utiliser le digital pour transmettre et former vos équipes sur le terrain
- Deciphering the complex characteristics of nanomedicines
- L’industrie pharma doit réduire sa trace carbone, ... le traitement d’air. Part 2
Calculation of greenhouse gas (GHG) emissions expressed in CO2eq of an open system (AinB) compared to a closed system equipped with isolators (AinD)
Due to its enormous complexity, the production of ATMP (Advanced Therapy Medicinal Products) has quite high costs, which mainly consist of direct costs (such as reagents, materials and products, clothing, personnel and quality control costs). However, this production has a significant impact also on indirect costs, which are related to qualifications and validations (e.g. media filling, cleaning and environmental controls) and to the whole structure, including energy costs, air conditioning costs, HVAC systems and environmental validation.
This article presents an analysis conducted to highlight opportunities to improve the environmental impact of the manufacturing of advanced therapy medicinal products (ATMPs), by comparing greenhouse gas (GHG) emissions expressed in CO2eq of a classic Grade B clean room with Grade A laminar flow cabinets (open system) versus a closed system equipped with isolators, considering energy requirements, carbon footprint and costs related to a 21-day production cycle.
1. Manufacturing of ATMPs
The European Regulation 1394/2007 defines advanced therapy medicinal products (ATMPs) as products used to restore, correct or modify physiological functions primarily through a pharmacological action.
The ATMP manufacturing process for clinical use must comply with the principles of good manufacturing practice (GMP) to determine how cell preparations are produced and controlled, from the collection and handling of raw materials to the processing of intermediate products and quality controls, storage, labeling, packaging and release.
GMP Annex 1 describes the appropriate classification required and assigned to pharmaceutical manufacturing areas, depending on the type of operations taking place in the facility:
- Grade D: Represents a clean area where the less critical stages of sterile manufacturing take place.
- Grade C: This area is accessible from Grade D and is associated with a clean area designated for less critical stages in the production of sterile products. Grade C requires higher air and particulate and microbiological surface quality than Grade D, while sharing similar temperature and relative humidity control.
- Grade B: For aseptic preparation and filling, this is the base environment for Grade A. Air pressure differences must be constantly monitored. Requires higher air and microbiological and particulate surface quality than Grade C, while sharing similar humidity and temperature control. Environmental contamination conditions must be constantly monitored.
- Grade A: an area of high-risk operations requiring aseptic conditions (e.g. filling, opening and closing of containers, such as vials or test tubes). These conditions are usually ensured by a homogeneous laminar airflow in the work environment and are monitored throughout the critical processes.
The guidelines also define two types of system in which aseptic production of drugs (in general and of ATMPs in particular) can take place:
- The open system is defined in general as an aseptic preparation and filling area in which the product is exposed to the environment and operator (for example when the product is handled in a laminar flow cabinet) ; in this case, according to the GMP guidelines, a grade A critical clean area inserted in a grade B background clean area is required (AinB).
- The closed system, on the other hand, involves the use of a physical barrier that absolutely separates the operator and the environment from the area in which the product is handled (Grade A Isolator); in this case, it is accepted that the background environment where the isolator is installed, is, always according to GMP guidelines, of Grade D (AinD).
- Due to its enormous complexity, the production of ATMPs has quite high costs, which mainly consist of direct costs, such as reagents, materials and products, garments, personnel and costs related to quality controls. However, this production has a significant impact also on indirect costs, which are related to qualifications and validations (e.g. media filling, cleaning and environmental controls) and to the entire structure, including energy costs, air conditioning costs, HVAC systems and environmental validation.
2. Methods
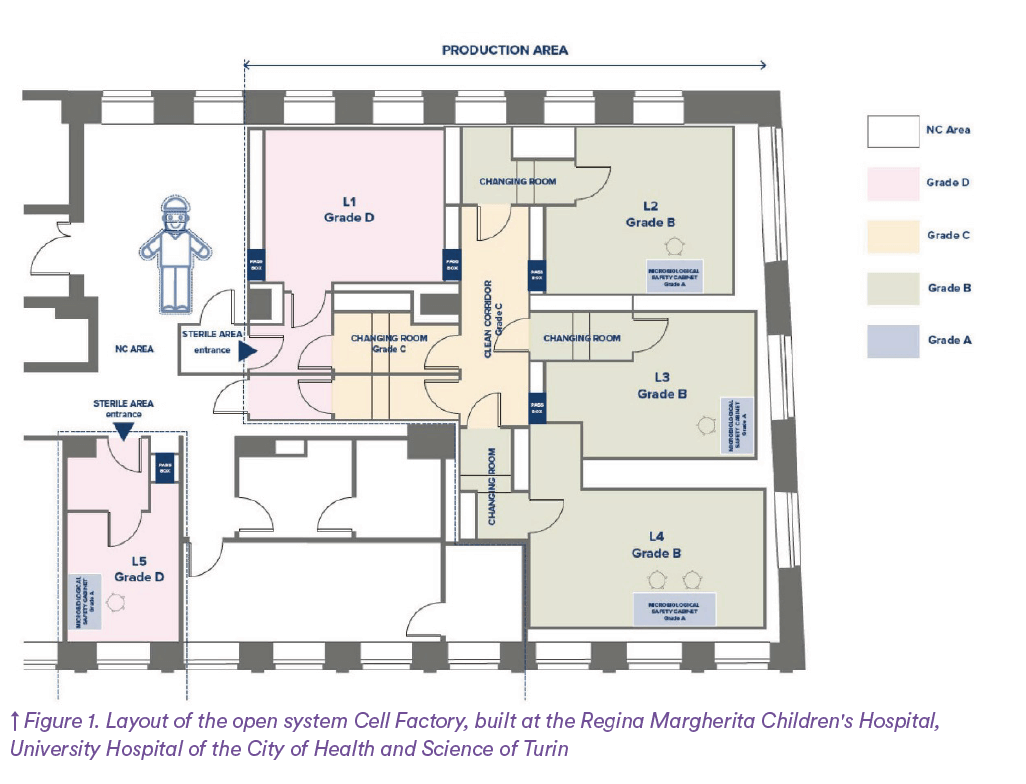
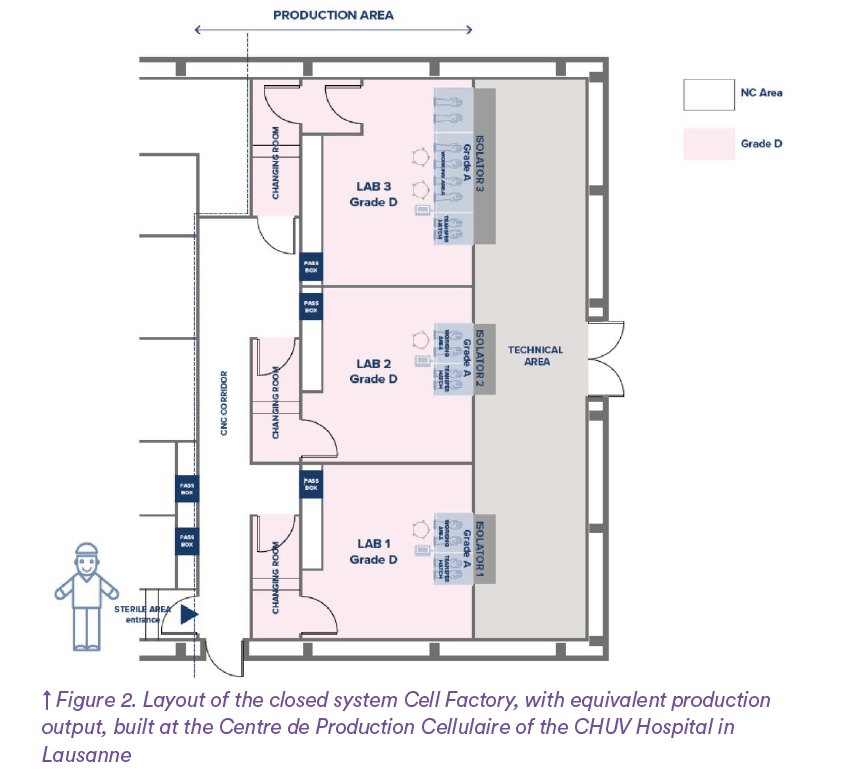
The analysis of the difference between open and closed systems was performed by comparing the Cell Factory of Regina Margherita (open system Figure 1) and the Centre de Production Cellulaire (CPC) located in Epalinges (closed system Figure 2) and belonging to the University Hospital of Lausanne (CHUV).
The following operating parameters were considered and collected for the analysis, both from the open system Cell Factory and the closed system Cell Factory:
- Annual energy supply for ventilation of the controlled environments (kWh);
- Annual energy supply for the installed equipment (kWh);
- Costs of the twenty-one-day production cycle (in EUR);
- Greenhouse gas emissions (in CO2eq);
- An estimate of the construction costs for the open and closed systems.
The energy costs related to each controlled environment in the open and closed system were calculated based on the different surfaces and environmental classifications of the two Cell factories and the related hourly air changes.
The total air volume changes for each controlled environment in both open of 271m² and closed system layouts (108m²) have been calculated based on the different surface areas of the two CFs and the relevant Grade air changes per hour:
- Grade B: 55 changes per hour and
- Grade D: 35 changes per hour.
3. Cost analysis
A production cycle of 21 days/cycle was considered in both the open and closed systems.
These costs, provided by the Regina Margherita hospital for the open system, were divided into indirect and direct costs as follows:
- Indirect costs for the structure (Lighting/electricity of the equipment ; Air conditioning/HVAC system ; Environmental validation; Annual qualification tools and products.)
- Indirect costs for production (Media filling for two operators ; CF cleaning (biocides + clothing) ; Qualification of the qualified operator’s dressing ; Qualification of the external operator’s dressing ; Material flow ; Environmental controls (monthly).
- Direct costs for production : Biocides ; Dressing ; Personnel (cost man/hour).
The data were measured in same season. However, the advantage using a closed system remains because the volume of fresh air which is thermally treated, at every cycle, is higher for the open system than for the closed system and therefore the energy consumed will be correspondently higher for the open system.
4. Greenhouse gas emission in (CO2eq)
The CO2eq footprint was obtained considering the impact category indicator (ISO 14040) for the production of 1 kWh in Italy (0.8), and multiplying by the difference in electricity consumption; for this characterization factor. The calculated value corresponds to 406.31 g/kWh CO2eq. This approach is normally implemented in the life cycle assessment (LCA) study of products and is strongly recommended by the ISO 14067 standard The costs for the construction of the infrastructure are related to the complexity and size of the structure. The analysis was performed considering the average prices/m² for this type of structure in Europe.
5. Results
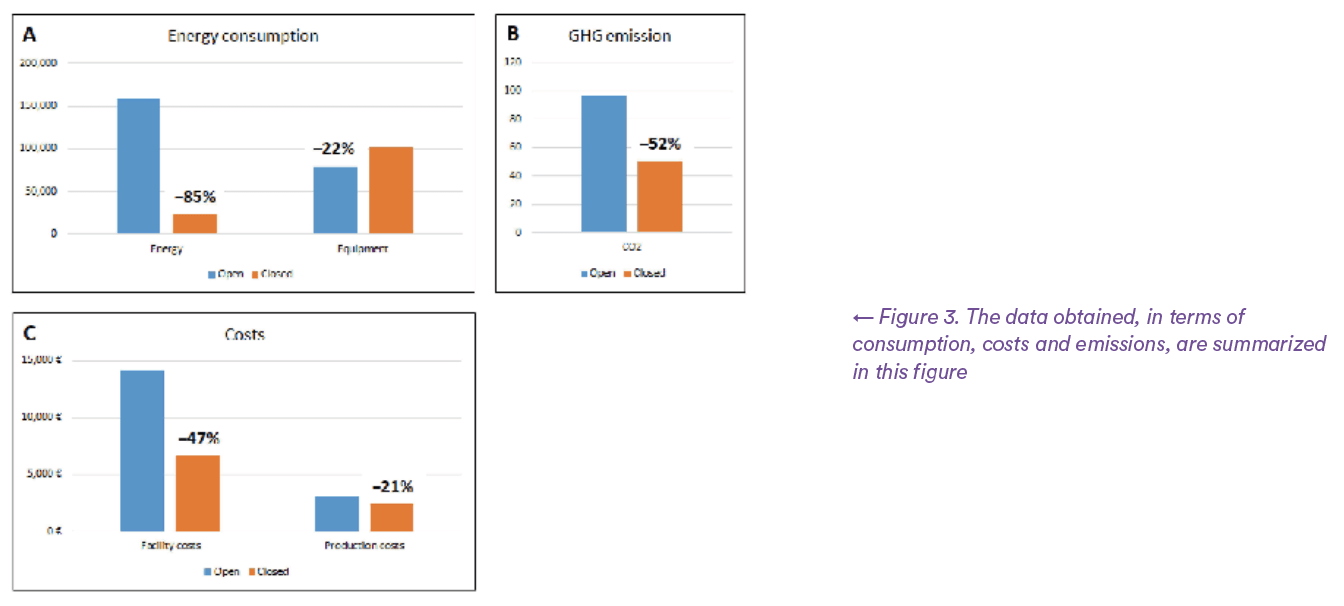
Based on the different surfaces of the controlled environments used in the two configurations and the related air changes to maintain the levels of particle control, the overall average daily energy consumption was calculated at 435 kWh/ day in the CF of Regina Margherita and 63.23 kWh/day in the closed system of CPC, with a saving of 85% on ventilation costs for the closed system compared to the open system.
The 21-day production cycle used at the CF of the open system involves three internal operators and two external operators, as well as quality control procedures.
This analysis shows that the energy savings achieved by using a closed system is 6,497 kWh/21 days of production process and that, consequently, the reduction of greenhouse gas emissions using closed systems is approximately 52% per process cycle compared to open systems. Paradoxically, but understandably, the energy consumption of the Grade A isolator used in the closed system is higher than the energy used by the laminar flow hoods installed in the open Grade B system for the critical Grade A area (13,671 kWh versus 7,174 kWh/process respectively).
However, comparing the total value of energy consumption calculated as the sum of the energy required for ventilation, distribution and treatment of air and instrumentation, we have shown that the energy required to complete a 21-day production cycle in an open system is significantly higher than that required by an equivalent production cycle in a closed system. The same applies, as seen below, to greenhouse gas emissions, which are higher for open systems than those generated by the use of closed systems. (see Figure 3).
Conclusions
Advanced therapy medicinal products (ATMPs) are transforming the treatment of many previously incurable diseases. However, their complex preparation, high production costs, and the need for compliance with Good Manufacturing Practices (GMP) make their use challenging and expensive. In this study, we have calculated the costs and the carbon footprint of ATMP production by comparing the production cycles in two different systems: an open AinB system and a closed AinD system, as outlined in EudraLex Vol.4, Annex 1 and Part IV.
Since biological drugs cannot be sterilized, they must be produced in sterile environments. These environments are maintained aseptic through strict control of particulate and microbiological contamination, which is achieved using ventilation systems. These systems filter, recirculate, and condition the air to ensure sterility. The traditional open AinB clean room system requires extensive air recirculation, making it highly energyintensive. In contrast, isolators offer a higher level of protection against product contamination and provide a safer working environment for operators.
We analyzed a hypothetical 21-day production cycle in both open and closed systems, demonstrating a reduction in production costs when using an isolator. Additionally, by employing an isolator in a Grade D room, the cost of disposable sterile cleanroom gowns is significantly lower compared to the expenses associated with an open system. Moreover, decontamination cycles in the closed system are designed and validated for smaller volumes, are automated (typically using hydrogen peroxide vaporization), and do not require specialized personnel. The validation of smaller environments in the AinD closed system processes is also notably less expensive and timeconsuming compared to the larger environments required in the AinB open systems, which, by definition, include Grade B, C, and D environments.
Finally, in this study, we analyzed the sustainability of a traditional cleanroom compared to a closed system equipped with isolators, specifically focusing on Greenhouse Gas (GHG) emissions expressed in CO2 equivalents (CO2eq), to identify opportunities for reducing the environmental impact of ATMP manufacturing.
The analysis revealed an energy saving of 6,497 kWh over the 21-day production cycle. As a result, the reduction in GHG emissions when using closed systems is approximately 52% per production cycle.
References
- EUR-Lex Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/ PDF/?uri=CELEX:32007R1394 (accessed on 8 May 2023).
- Silva, D.N.; Chrobok, M.; Ahlén, G.; Blomberg, P.; Sällberg, M.; Pasetto, A. ATMP Development and Pre-GMP Environment in Academia: A Safety Net for Early Cell and Gene Therapy Development and Manufacturing. Immuno-Oncol. Technol. 2022,16, 100099.
- European Medicines Agency. Advanced Therapy Medicinal Products: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory/ overview/advanced-therapy-medicinal-products-overview (accessed on 8 May 2023).
- EudraLex-Volume 4–Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Available online: https:// health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 8 May 2023).
- Eudralex Volume 4 Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Annex 1. Manufacture of Sterile Medicinal Products. Available online: https://health.ec.europa.eu/document/download/e05af55b-38e9-42bf-8495-194bbf0b9262_ en?filename=20220825_gmp-an1_en_0.pdf (accessed on 8 May 2023).
- Zanini, C.; Severina, F.; Lando, G.; Fanizza, C.; Cesana, E.; Desidera, D.; Bonifacio, M. Good design practices for an integrated containment and production system for advanced therapies. Biotechnol. Bioeng. 2020, 117, 2319–2330. [CrossRef] [PubMed]
- Kropp, M.; Harmening, N.; Bascuas, T.; Johnen, S.; De Clerck, E.; Fernández, V.; Ronchetti, M.; Cadossi, R.; Zanini, C.; Scherman, D.; et al. GMPGrade Manufacturing and Quality Control of a Non-Virally Engineered Advanced Therapy Medicinal Product for Personalized Treatment of AgeRelated Macular Degeneration. Biomedicines 2022, 10, 2777. [CrossRef] [PubMed]
- Chemili, M.; Laurent, A.; Scaletta, C.;Waselle, L.; Simon, J.P.; Michetti, M.; Brunet, J.F.; Flahaut, M.; Hirt-Burri, N.; Raffoul,W.; et al. Burn Center Organization and Cellular Therapy Integration: Managing Risks and Costs. J. Burn. Care Res. 2021, 42, 911–924.
Partager l’article

Ivana FERRERO – Ospedale Infantile Regina Margherita Torino