A3P Switzerland Congress
Annex1 GMP Eu
Lieu
Mövenpick Hôtel Lausanne
Date
March, 11&12
Save The Date !

FORMAT
Conferences, Partners Workshops, Exhibition
Partager
The A3P Switzerland congress in March 2025 will delve into the interpretation and application of the new Annex 1 in Switzerland, with a focus on sharing practical insights and experiences from the industry. Since the publication of Annex 1 Manufacture of Sterile Medicinal Products and its implementation on August 23 with PICs PE 009-17, the pharmaceutical and biotechnology sectors have been faced significant changes in process design, aseptic practices and contamination control requirements. This forum will provide a unique opportunity to discuss Swissmedic’s interpretation of these new requirements and to examine the strategies and challenges encountered by industry leaders in their implementation efforts. Participants will gain insight into compliance expectations and innovative approaches to meet the stringent standards now required for the manufacture of sterile and low microbial load medicinal products.
Tuesday, March 11
Wednesday, March 12

Tuesday, March 11
Session 1
Session 2

Wednesday, March 12
Session 3
Session 4
Map of the exhibition
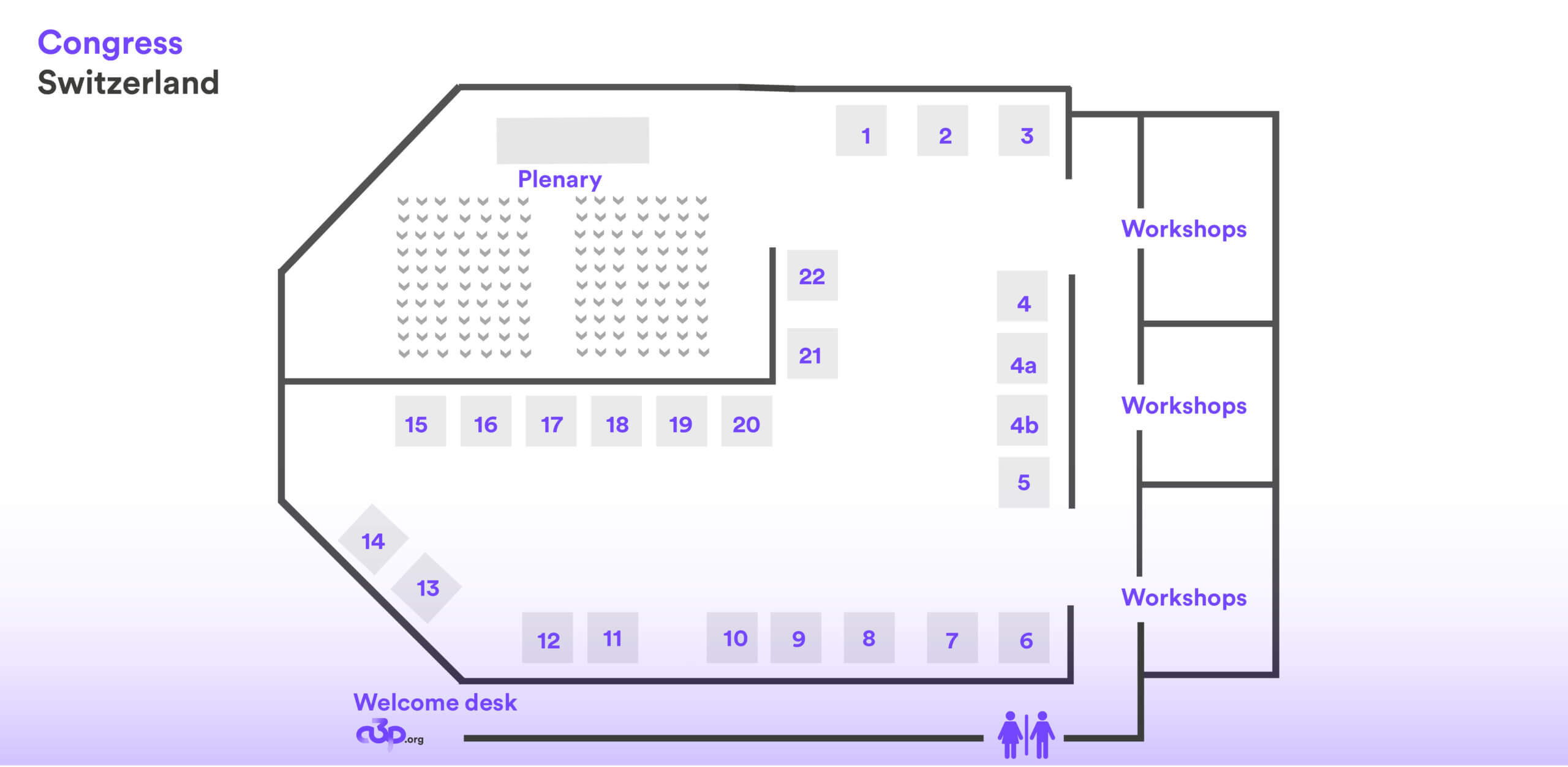
| Société | N° Stand | Société | N° Stand | |
| PHARMAMEDIA DR.MÜLLER | 1 | SYMBIOSE ENVIRONNEMENT | 11 | |
| PHARMTEC | 2 | JCE BIOTECHNOLOGY | 12 | |
| SYNEXIN | 3 | SIEMENS | 13 | |
| STERIS | 4 | PACOSPHARM | 14 | |
| SALAMANDERU | 4a | ACTEMIUM | 15 | |
| BECKMAN COULTER | 4b | GETINGE | 16 | |
| SOURCIN | 5 | NOVATEK | 17 | |
| BD LIFE SCIENCES | 6 | LAPORTE EURO | 18 | |
| EREA | 7 | ACD SWISS | 19 | |
| DEVEA | 8 | ELIS CLEANROOM | 20 | |
| ASSOCIATES OF CAPE COD | 9 | ICARE | 21 | |
| AM INSTRUMENTS | 10 | SOLIDFOG | 22 |
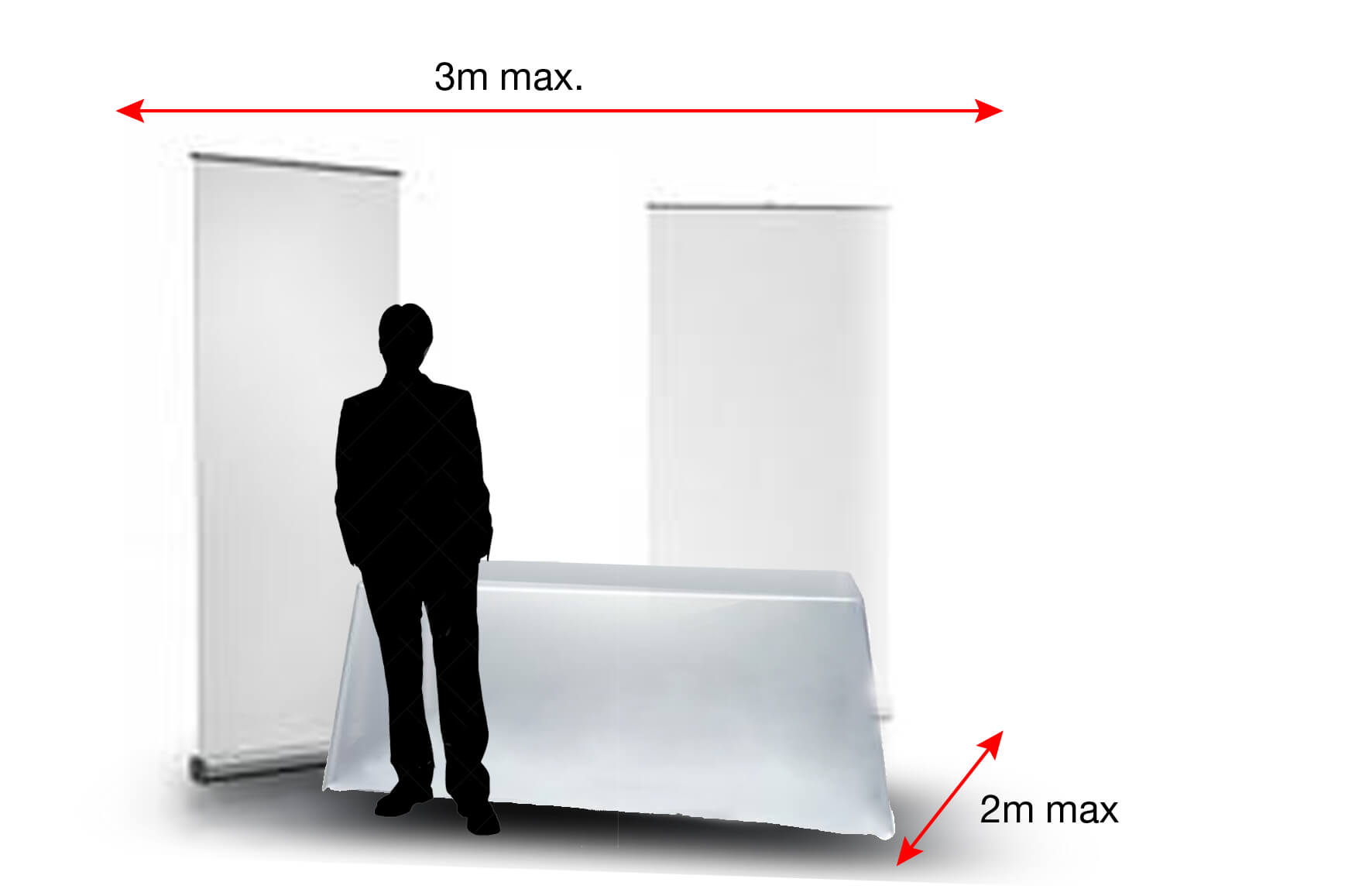
Services included with the Table Top
- A dedicated exhibition space where you can set up your products and communication tools: umbrella stand, roll-up, display, product, etc. Basic equipment including a 180×80 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker
- Breaks and meals as indicated in the program
- Unlimited WiFi
- A badge giving you free access to the entire technical and scientific program
- List of event participants
Registration fee: 1 240€excluding VAT (20% VAT) + A3P membership required* *additional costs to be expected if you are not yet a member.
To find out if you are a member, please contact us.
For prices, please see: https://www.a3p.org/en/membership/
Contact
You can contact Victoria PALOMBI :
Téléphone :+337 58 68 94 49
Email : vpalombi@a3pservices.com
Courrier : A3P, 30 rue Pré-Gaudry – Lyon 69007
Code promo hôtel :
Pour réserver cliquer ici
Ajouter dans la case « Special rates » le code « MOV1 » dans la partie « Preferential code » et appuyer sur « Submit »
Accessibilité



