Sommaire
- L’intérêt de la désinfection automatisée des surfaces d’un RABS par decontamination aérienne
- Utilisation d’isolateurs individuels pour la thérapie cellulaire autologue
- Calculation of greenhouse gas (GHG) emissions expressed in CO2eq of an open system (AinB) compared to a closed system equipped with isolators (AinD)
- Exigences croisées de la norme ISO 13408-2 et de l’Annexe 1
- Employing Conductivity Measurements for On-site Residue Quantification
- LEAN. Utiliser le digital pour transmettre et former vos équipes sur le terrain
- Deciphering the complex characteristics of nanomedicines
- L’industrie pharma doit réduire sa trace carbone, ... le traitement d’air. Part 2
Deciphering the complex characteristics of nanomedicines
In the last decades, nanomedicine, the medical application of nanotechnology, has offered many new opportunities in the medical field. Nanomedicines are mainly injectable formulations based on nanoparticles which are used for diagnostic, therapeutic or preventive purposes. Nanoparticles consist of biocompatible inactive components (e.g., lipids, polymers, metal oxides) and may contain therapeutic payloads and/or targeting moieties depending on the application.

1. Introduction to nanomedicines
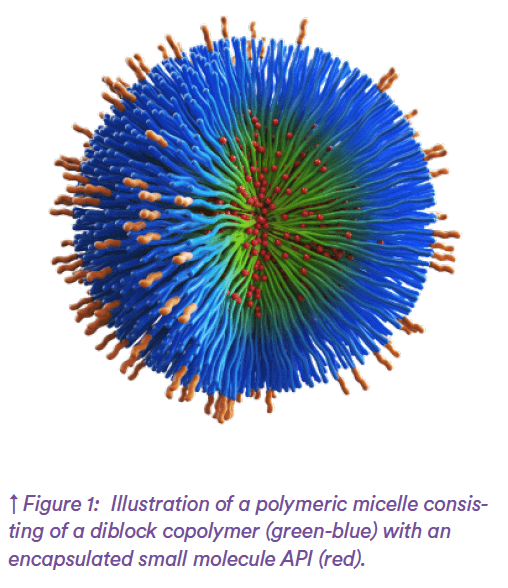
Nanomedicines can improve MRI imaging contrast or enhance the efficacy of active pharmaceutical ingredients (APIs) when they are used as drug delivery vehicles. Efficacy is improved by enhancing the solubility of small molecule drugs, by increasing the stability of sensitive therapeutical agents (e.g., proteins, nucleic acids) or by delivering APIs directly to targeted cells. Different structures and morphologies of nanomedicines can be designed including core- shell structures, liposomes and micelles (Figure 1). The composition and characteristics of the nanoparticles – such as size, morphology, and surface properties – can be tailored to meet specific needs.
Since the approval of the first liposomal drug product Doxil® (doxorubicin HCl liposome injection) in 1995, various other nanomedicines have been approved. The most notable recent examples are the COVID-19 vaccines from Pfizer-BioNTech (Corminaty®) and Moderna (Spikevax®). These vaccines are based on the mRNA- lipid nanoparticle (mRNA-LNP) technology which allowed for the rapid development and large-scale production of highly effective and safe vaccines during the COVID-19 pandemic.
2. Critical quality attributes
Nanomedicines have various chemical, physical and biological attributes due to their multi-component compositions and complex structures1. In addition to the composition of nanoparticle components and their impurities, properties such as particle size, morphology, drug release kinetics, surface properties, and interaction with biological systems may affect efficacy or safety. Developing and validating analytical methods for all possible nanoparticle properties during early-stage development is unrealistic because it significantly increases the cost and time-to-market.
A cost- and time-effective strategy is to identify the critical quality attributes (CQAs) early in development. CQAs are the properties that directly impact the nanomedicine’s safety and efficacy. Phase-appropriate analytical methods can then be developed and validated. As the product progresses through clinical phases, more detailed and accurate characterization is required. This can be achieved by including additional testing and more sophisticated analytical techniques.
3. Challenges in analytical method development
3.1 Chemical and structural complexity
The complexity of nanomedicines poses a challenge in analytical method development. To quantify and determine purity of nanoparticle components (e.g., APIs, lipids, target moieties, etc.), they must be extracted from the complex matrix and analyzed by chromatographic or spectrometric techniques. Developing such assays is challenging due to the required sample preparation, particularly when the components are covalently bound to each other. Quantification of the unencapsulated API is also required and is usually achieved by laborious sample preparation procedures such as solid-phase extraction or centrifugal ultrafiltration.
Particle size is a CQA in most cases as it influences pharmacokinetics and efficacy. In addition, not all nanoparticles within a formulation are the same size. A certain degree of size polydispersity, originating from the manufacturing process, must also be quantified and controlled. Changes in average particle size or polydispersity over time may indicate degradation, dissociation, or aggregation of nanoparticles. Therefore, precise and accurate methods to deter- mine particle size distribution are essential for ensuring stability and batch-to- batch consistency. Typically, particle size is measured by dynamic light scattering (DLS) which has certain limitations (low resolution, biased towards larger sizes).
Other characteristics such as shape and morphology may also be critical. These attributes are typically measured by microscopic techniques such as trans- mission electron microscopy (TEM), which require proper method development to ensure the structure remains unaltered during sample preparation.
Furthermore, nanomedicines are often designed to interact with their environ- ment in specific ways, requiring the development of functional assays. Examples include determining the magnetic properties of iron oxide nanoparticles, the release rate of an encapsulated drug, or the interactions with plasma proteins or immune cells.
3.2 Limited understanding in early phases
Early development is typically focused on commonly encountered CQAs and their testing with standard analytical techniques. However, the exact critical properties are often poorly understood during these early stages, making method development difficult. Over time, as more information becomes available from process development, stability studies, and characterization with sophisticated techniques, understanding improves. This evolving understanding may reveal that early-stage methods are not suitable to accurately determine CQAs such as size distribution or specific degradation products. Consequently, new methods and re-validations are often required, leading to unforeseen expenses and potential delays. In addition, the complex manufacturing processes of nanomedicines can lead to small but critical batch-to-batch variations. These variations may go undetected with early-stage methods which typically focus mainly on identifying gross defects rather than subtle differences.
3.3 Evolving regulatory standards
Nanomedicines, due to their complexity and novel properties, undergo strict regulatory review. Agencies expect that CQAs for release and stability testing are well-defined and that analytical methods are properly developed and validated at every stage. However, there are currently limited global standards to guide manu- facturers through this process2. Each nanomedicine is unique, with variations in chemical composition and behavior, requiring specifications and analytical methods to be adapted for each formulation. Detailed standard protocols can only be created for subcategories (e.g., mRNA-LNPs) or individual formulations.
Currently, no comprehensive guidance documents exist for validating tech- niques such as DLS or size-exclusion chromatography. Similarly, no analytical reference materials tailored to nanomedicines are available for testing accuracy. For example, NIST-certified polystyrene standards, typically used as size standards for DLS, differ from nanomedicines in chemical composition and properties. This lack of standardized methods, validation procedures, and product-specific certified reference materials poses significant hurdles for quality control and characterization.
Several international and regional regulatory organizations are working on the standardization of nanomedicine practices. Technical committees on nanotechnology have been established by ISO and ASTM, and a dedicated research laboratory by the European Commission (part of Joint Research Centre). Additionally, EMA and FDA have issued general guidance documents on the manufacturing and control of drug products containing nanomaterials and liposomes. The United States Pharmacopeia and the European Pharmacopoeia have also established dedicated working groups to develop chapters and mono- graphs related to nanomedicine. While these initiatives aim to facilitate drug development in the long term, evolving regulatory standards in the short term create additional challenges. Methods already developed may require updates and re-validation to maintain regulatory compliance as new standards emerge.
4. Overcoming challenges and the critical role of advanced analytical techniques
To overcome the aforementioned challenges, investment in state-of-the-art infrastructure and training of personnel is crucial. Therefore, drug development of nanomedicines typically takes place in dedicated departments or Contract Development and Manufacturing Organizations (CDMOs) specialized in this field. These groups have the expertise, infrastructure, and regulatory knowledge to deal with the complexity and versatility of these novel modalities. They also adopt a flexible phase-appropriate analytical strategy, enabling them to adapt methods to specific requirements, unexpected results, or evolving regulatory standards at any stage. Engagement with nanomedicine-related initiatives from regulatory agencies or standardization bodies (FDA, EMA, pharmacopoeias, ISO, etc.) is necessary to meet regulatory expectations.
Another key strategy to overcome analytical challenges is to adopt sophisticated analytical techniques, not only in later stages where detailed characterization is required, but also early in development. These techniques better capture the complexity of these products, providing more accurate results and comprehensive characterization. One such technique is Asymmetric Flow Field-Flow Fractionation (AF4) coupled with multiple online detectors, which we elaborate on further in this article.
The particle size distribution of nano- medicines is typically measured by (single angle) DLS but this method has several limitations. First, it has low resolution as it cannot discriminate between sizes that differ by a factor of two. For example, a formulation containing a significant fraction of dimers and trimers may appear as having a unimodal size distribution in DLS, leading to incorrect conclusions. Second, DLS is biased towards larger sizes, which scatter light more intensely. When analyzing samples with high polydispersity, large particles may ‘overshadow’ the signal of smaller particles, rendering them effectively invisible. Third, DLS does not provide information on other critical properties such as morphology, shape, or chemical composition. While Nano-particle Tracking Analysis (NTA) offers higher resolution, it has a narrower size range and lower accuracy as it measures only a small fraction of the sample. TEM is a valuable tool to visualize nanoparticle morphology and shape, but size measurements are less accurate due to potential alterations during sample preparation and the limited number of particles analyzed.
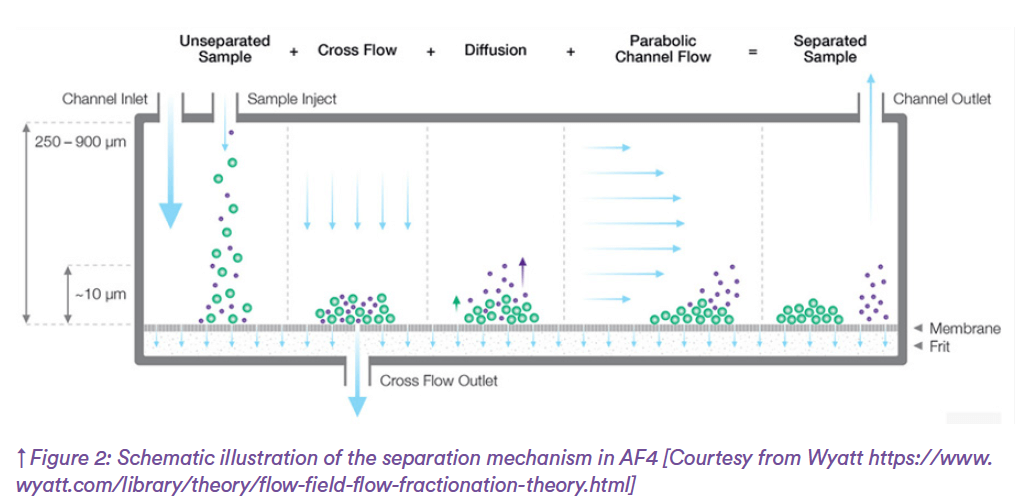
AF4 is an analytical technique that separates nanoparticles based on size within a channel through two key forces: a laminar flow with a parabolic profile applied along the channel and a cross-flow applied through an ultrafiltration membrane (Figure 2). The nanoparticles are retained by the membrane while they diffuse, entering different flow streams of the parabolic flow profile and, consequently, separating based on their hydro-dynamic size. In contrast to size-exclusion chromatography, AF4 does not have a packing material which results in higher sample recovery and lower shear forces, and therefore more accurate results. AF4 has a broad size range (5 – 500 nm) and has been used extensively to characterize macromolecules, nanoparticles and other complex biological systems (e.g., blood plasma, viruses).
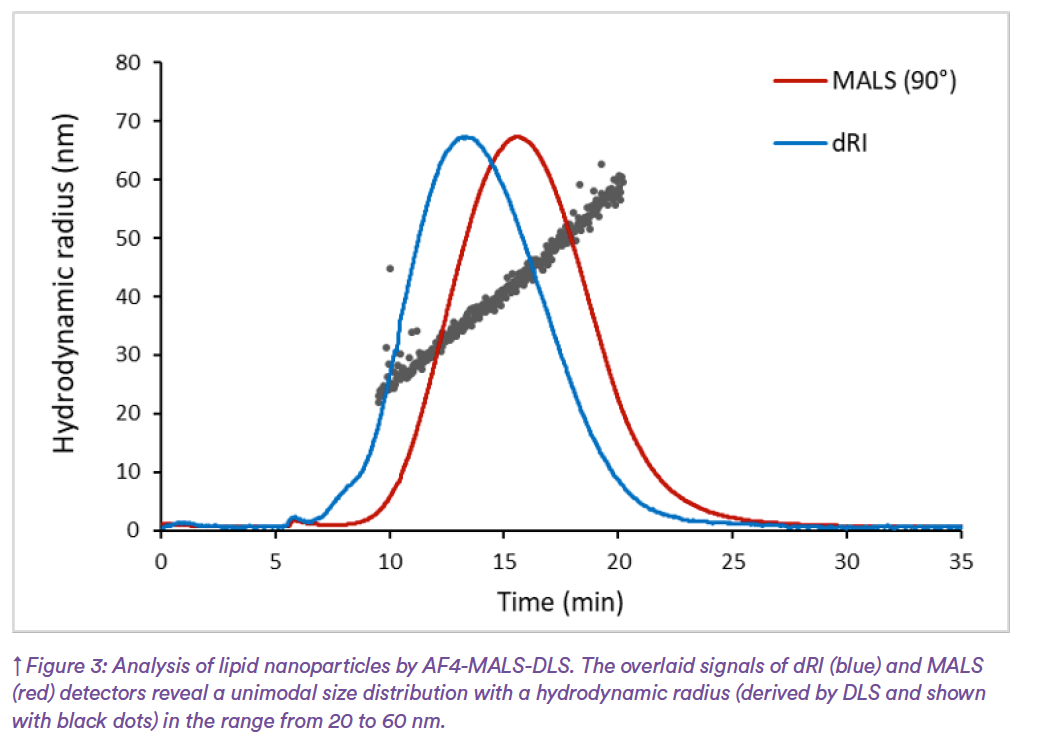
When AF4 is coupled with DLS (AF4- DLS) and a concentration detector, such as UV or differential refractive index (dRI), accurate particle size distribution based on the hydrodynamic size (Rh) can be obtained (Figure 3). This configuration overcomes the limitations of batch-mode DLS as the particles are first separated with high resolution and then detected by DLS. In addition, AF4 coupled to multi-angle light scattering (AF4-MALS) and concentration detectors provides information on the molar mass distribution and size distribution based on the radius of gyration (Rg) (Figure 3). Combining both light scattering detectors (AF4-MALS-DLS), offers indirect insights into morphology and shape via the shape factor (Rg/Rh)3. For compact spheres this ratio equals 0.78, for empty liposomes it equals 1, and for elongated particles it is >1. The chemical composition may also be derived from UV and dRI detectors. Thus, AF4-MALS-DLS can be used not only to determine particle size distribution and nanoparticle stability over time (degradation or aggregation), but also morphology, free components, and drug loading. Fractions of different sizes can also be collected and measured with other techniques to investigate size-dependent variations in chemical composition, surface charge, or other properties (4). Furthermore, nanomedicines are often designed to behave in specific ways in biological systems such as degrading at a specific rate to release the API or avoiding unwanted interactions like protein corona formation on the surface. These interactions are hard to predict using standard in vitro assays but can be evaluated using AF4-MALS-DLS5.
Conclusion
Phase-appropriate analytical development of nanomedicines is challenging due to their chemical and structural complexity, the limited understanding of their CQAs in early stages and the evolving regulatory requirements. These challenges can be addressed by investing in technological innovation early in development, particularly in analytical techniques capable of simultaneously measuring multiple CQAs. Such techniques enable more in-depth characterization and robust batch-to-batch comparisons. One example is AF4-MALS-DLS which can measure particle size, molar mass, stability, morphology, and drug loading. It can also evaluate the fate of nanomedicines in relevant biologic fluids, such as blood plasma, providing valuable insights into pharmacokinetics.
References
1. Fan et al., Analytical characterization of liposomes and other lipid nanoparticles for drug delivery, J Pharm Biomed Anal,192 (2021), 113642
2. Giordani et al., Liposomes characterization for market approval as pharmaceutical products: Analytical methods, guidelines and standardized protocols, J Pharm Biomed Anal, 236 (2023), 115751
3. Parot et al., Quality assessment of LNP-RNA therapeutics with orthogonal analytical techniques, J Control Release, 367 (2024), pp. 385-401
4. Ansar and Mudalige, Characterization of doxorubicin liposomal formulations for size-based distribution of drug and excipients
using asymmetric-flow field-flow fractionation (AF4) and liquid chromatography-mass spectrometry (LC-MS), Int. J. Pharm, 574 (2020), 118906
5. Caputo et al., Asymmetric-flow field-flow fractionation for measuring particle size, drug loading and (in)stability of nanopharmaceuticals, J Chromatogr A, 1635 (2021), 461767
Partager l’article

Maria MARIOLI – ARDENA