Sommaire
- Developments in Container Closure Integrity Testing of Lyophilised Product
- Development of Platform Processes for the Manufacture of Biopharmaceuticals
- Minimiser les risques de la chaîne de la valeur dans le domaine de la santé
- Propositions de modifications spécifiques de l’Annexe 1
- La qualité et la stérilité du produit fini d’un process B.F.S.
- Création d’un Groupement d’Intérêt Commun A3P B.F.S. ?
- Fully automated Particle Inspection in Blow Fill Seal Containers – A new approach
- Principes du lavage automatique et erreurs courantes
- Four strategies to improve competitiveness in the pharmaceutical and medical device industries
- Management – Quelques pensées sur la direction de sites et le leadership, Partage d’expérience et de petits trucs…
A platform process is a production process suitable for the manufacture of a group of related products in a defined production system. At Lonza one of our technology offerings is a platform process for the production of therapeutic proteins in Chinese hamster ovary (CHO) cells.
Platform processes have a number of advantages over the development of a bespoke process for each new product.
• Platform processes allow rapid, cost-effective project progression, because use of scale-down models of the platform process during cellline screening enables the selection of a production cell line that fits the platform process. This removes the need for time and resource intensive process development activities.
• Platform processes enable deep knowledge of the process design space to be accumulated rapidly, because many products use the same process. Knowledge acquired with one product can be used to trouble shoot problems with another product.
• Platform processes provide assurance of saleability, as early products demonstrate the capability and define the best approach.
At Lonza our platform processes are a key offering, allowing our customers to rapidly generate material for clinical trials in a process that is well characterised, safe in the knowledge that the process has been scaled up for many previous products.
Principles
Fundamental to the design of any new platform process should be the principles of Quality by Design (QbD). ICH Q8/9/10/11 defines QbD as, A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. We will explore what this looks like by reference to the development of Lonza’s current platform process and ongoing activities in the development of the next-generation platform process.
Development of Lonza’s Current Platform Process
Prior to commencement of work on the current platform process a thorough review of the previous platform process was undertaken. This sought to identify areas for improvement. Based on this review a list of objectives for the new process was drawn up.
One key objective was the development of a new cell culture medium (CCM). The previous platform process used a commercially available medium. The formulation of that medium was proprietary. This limited:
• Our ability to characterise a key subspace of the process design space.
• Our ability to trouble shoot problems associated with the medium.
• Our ability to control the raw materials and process used in the manufacture of the medium.
The CCM had its own list of objectives based around quality, compatibility, robustness, scalability and productivity.
The CCM was designed using a knowledgedriven, bottom-up approach. Over 65 components were selected for the medium based on:
• Suitability for cell culture
• Suitability for use in preparation of an injectable product (low toxicity and low likely hood of carrying advantageous agents)
• Low intrinsic batch-to-batch variability
• Stability in powder and liquid formulation
• Chemical compatibility
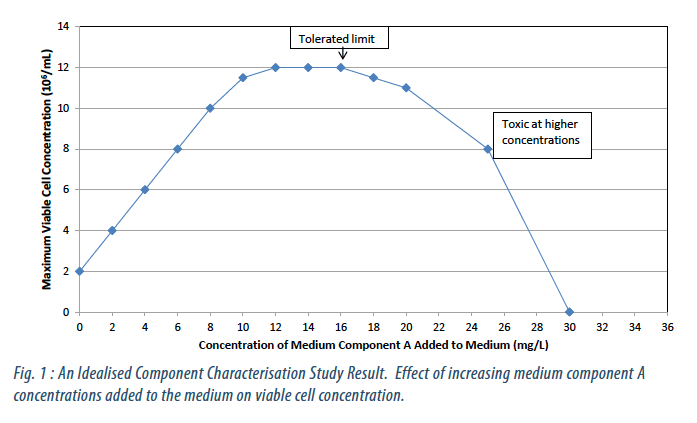
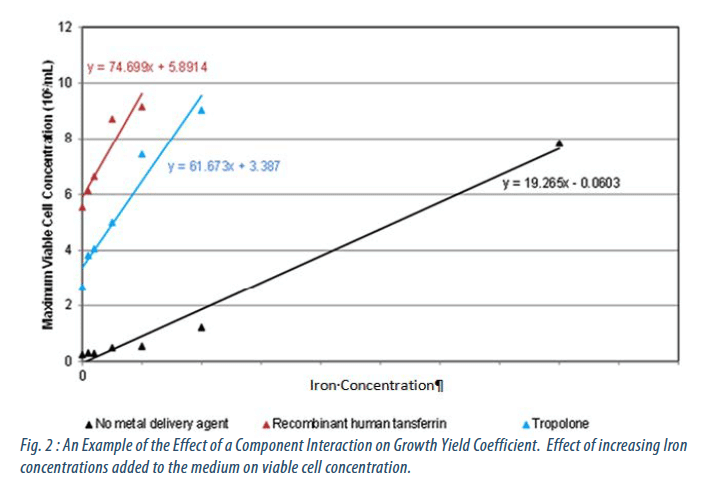
The performance of these components was characterised individually and, where appropriate, in combination. From this yield coefficients, limits of tolerance and component interactions were determined (Figures 1 and 2).
In parallel with development of the CCM a concentrated feed system was developed minimising feed volume to enable scale-up into a range of bioreactor formats at large scale. Again there was a strong emphasis of QbD, particularly in ensuring the chemical compatibility of the concentrated feeds.
Following development the scalability of the process was tested in a GMP facility prior to roll out to customer projects.
Lonza’s current platform process has proved very successful.
• Used at Lonza for the GMP manufacture of 10’s of products
• Titres in GMP up to 8 g/L
• Proven scalability to 20,000 L
• Successfully used by GS licensees around the world.
Development of Lonza’s Next-Generation Platform Process
In 2012 Lonza launched the GS Xceed™ Gene Expression System. One of the new components was a new CHO host cell line.
The new host cell line fitted the current platform process well. However, the arrival of the new host cell line was a good opportunity to review the design space of the platform process and update it to maximise the potential of the new host cell line and to keep up with improvements in the state of the art. The next generation of the platform process is under development.
The development of the next-generation platform process follows the same principles as the current process. However, since the development of the current process the expectations around the implementation of QbD have crystallised. Some of the differences of note are discussed below.
Review of the historical data for the current platform process has made use of multivariate data analysis tools, such as principle component analysis to facilitate understanding of behaviours observed in the process.
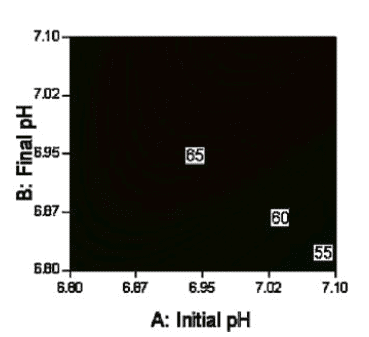
The development and characterisation of the next-generation process has focussed much more on the use of Design of Experiment (DoE) to develop the process and characterise the process design space. This allows better quantitative understanding of interactions between process parameters. An example of an interaction between process parameters with an impact on product characteristics is given in Figure 3.
The use of DoE has been facilitated by the availability of automated, miniature, highthroughput bioreactor systems that enable far more conditions to be evaluated in each experiment.
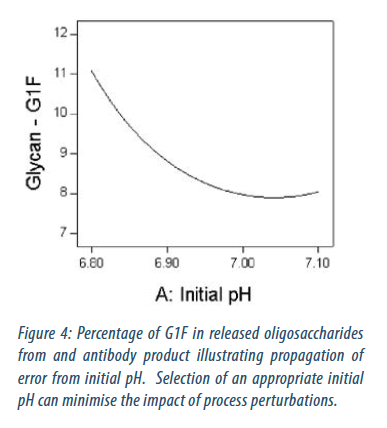
The use of DoE has also enabled propagation of error tools to be applied, enabling quantification of the robustness of the process to perturbations in process parameters. An example of how propagation of error from process control to product characteristics is given in Figure 4. At an initial pH of 7.05 assuming process variability of +/- 0.05 the predicted G1F is 8.0 +/- 0.1 %. At an initial pH of 6.85 assuming process variability of +/- 0.05 the predicted G1F is 9.9 +/- 1.1 %. By selecting an appropriate Initial pH the sensitivity of the process to perturbations in initial pH can be minimised. Moreover, because the propagation of error analysis is performed as part of the DoE the resulting model can be used to simultaneously optimise both the process outputs and the propagation of error from the process parameters to the process outputs.
Lonza’s current platform process was designed with a strong emphasis on achieving intrinsic process robustness. However, for the nextgeneration platform process there has also been a strong emphasis on process simplicity. Process simplicity is a complementary aspect of process robustness, leading to a reduction in execution errors. The requirements of intrinsic process robustness and process robustness through simplicity are often opposed. Finding appropriate compromises or routes around these contradictory requirements brings new challenges to the process developer.
Conclusions
Good platform processes have always embodied the principles laid down by the regulatory authorities in QbD. Lonza’s current platform process has achieved great success through the application of these principles. Over time the tools for implementing the principles of QbD have become more sophisticated. The development of the nextgeneration platform processes is taking advantage of many of these new tools to increase the quality of the offering to our license holders.