Sommaire
- L’intérêt de la désinfection automatisée des surfaces d’un RABS par decontamination aérienne
- Utilisation d’isolateurs individuels pour la thérapie cellulaire autologue
- Calculation of greenhouse gas (GHG) emissions expressed in CO2eq of an open system (AinB) compared to a closed system equipped with isolators (AinD)
- Exigences croisées de la norme ISO 13408-2 et de l’Annexe 1
- Employing Conductivity Measurements for On-site Residue Quantification
- LEAN. Utiliser le digital pour transmettre et former vos équipes sur le terrain
- Deciphering the complex characteristics of nanomedicines
- L’industrie pharma doit réduire sa trace carbone, ... le traitement d’air. Part 2
Employing Conductivity Measurements for On-site Residue Quantification
After a long revision process the update to EU GMP Annex 1 was released in August 2022, and highlighted residue management as an important topic.
As per Annex 11, USP Chapter <1072> 2 and PDA Journal 3 best practice guidance for cleanrooms guide that surface residues need to be either managed, removed or reduced to an acceptable level. The recent Annex
1 update contains specific guidance and links residue management as part of the cleaning step. The EU GMP Annex 1 states in chapter 4.33 : “The disinfection of cleanrooms is particularly important. They should be cleaned and disinfected thoroughly in accordance with a written programme. […] Cleaning programmes should effectively remove disinfectant residues. […].”


1. What is a residue?
Residues on surfaces are not clearly defined in Annex 1. They can be very different in nature and could be dust, soil, dirt or manufactured product residues. Residues can come from cleaning agent and disinfectants used during the cleaning and disinfection process and are often overlooked or underestimated.
From a chemical perspective, the residues remaining on a surface from the use of detergents or disinfectants will be dependent on the formulation of the product but are mainly non-volatile, ionic ingredients.
These will accumulate on surfaces after product application and the amount left behind is dependent on the detergent of disinfectant formulation, and the application technique (spraying, wiping, or mopping) especially when the product is applied multiple times without any removal step.
It is important to consider and understand that the evaluation of residue build up, by visual assessment, is subjective. The visibility of residues depends on many parameters such as the light conditions, the surface type, the application technique, and product formulations.
2. What are the risks and how to manage the risk mitigation?
Any risk to the manufacturing process should be identified using risk analysis and mitigation actions assigned and assessed as part of the Contamination Control Strategy (CCS). A CCS is a combination of ongoing product and process risk assessments, in line with ICH Q9*, linked to the Quality Management System (QMS). The CCS should establish robust assurance of contamination prevention (and considering all types of contaminants including residues).
→ Evaluation of the Health and Safety risks
The principle Health and safety concerns when using detergents/cleaning agents and disinfectants is the risk they can pose to the operators applying them. These can be exposure risks and physical risks posed from their application.
Each detergent and disinfectant has, by nature, a level of toxicity, particularly true for disinfectants which are toxic to living microorganisms. However, these products should be safe for operators to use when used and applied according to the product label instructions and when adhering to any appropriate safety measures described in the Safety Data Sheet requirements.
Using different products (for example, different disinfectant types) in rotation (a regulatory requirement) can potentially create chemical interactions. An example is a Chlorine based product being applied after an acidic phenolic based product which in some circumstances can cause toxic Chlorine gas to be generated).
A physical risk that can be caused by the build up of disinfectant residues or interaction of residues from multiple products is sticky or slippery floors. In addition to the risk of slips, trips, and falls for personnel from slippery floors, sticky floors may cause the accumulation of dirt and debris. These could be difficult to remove and increase the risk of cross contamination.
In addition to the risks to operators applying detergents/cleaning agents and disinfectants, we also need to be mindful that residues that are not adequately managed may accumulate on surfaces. This presents an increased risk that these could migrate by various means into the pharmaceutical products being manufactured in the area. Their detection in a finished product could result in batch rejection, or potentially unanticipated affects on the pharmaceutical product itself.
→ Evaluation of the risks to the premises
A common source of facility damage is due to the mismanagement of residues. It is important to consider that residues should not only be removed from cleanroom surfaces where the residue is visibly apparent, but from every surface.
These residues, if not managed proactively, can cause degradation to the facility over time. Issues such as corrosion, discoloration, reduction of the epoxy sheen or delamination of surface layers can occur. This can result in high repair and rebuild costs.
→ Evaluation of the risks on disinfectant efficacy
Residue build up can, by the accumulation of multiple layers of disinfectant, make the surface inaccessible to subsequent disinfectant being applied – potentially limiting its full effectiveness. It cannot be forgotten that the physio-chemical interactions between products can also simply inactivate the disinfectant.
A hidden impact sometimes overlooked is the impact on environmental monitoring (EM). Accumulation of disinfectant residues on a surface, transferred to swabs or contact plates during sampling may have an inhibiting effect on the organisms recovered, even where neutralizers have been added in the EM culture media. Thus the EM results generated can be underestimated, and may even give false negative results (masking effect).
Evaluation and mitigation of the risk needs to be a part of the CCS, using the common risk evaluation tools and with the help and support of the experts from other departments.
To assess the levels of detergent and disinfectant residues left on a surface after application, determine how quickly they accumulate and determine the best way to remove them it is helpful to have a technique to measure and quantify them.
→ Residue Measurement and Best Practice Removal Techniques
Various methods and techniques are available to quantify the number of residues. From complex analytical methods such as Liquid or Gas-chromatography to simpler methods such as UV, IR, or Conductivity. The benefit of being able to use a simpler method is that it can give you an instant result using a portable device in the environment. In particular, the conductivity method is a practical tool to assess and quantify residues in real-time.
3. Measurement Method
1. To measure conductivity, a standard portable conductivity meter device is a relatively simple and accessible tool (easy to handle, easy to calibrate and to maintain).
2. For the sampling method, a wetted wipe is used to remove residues from the test surface and immediate measurement of the residues from the wipe in aqueous solution using a Conductivity meter.
3. Selection of high purity water: Purified Water or WFI (WFI is the best option) and a low particle wipe (e.g. 100% Polyester knitted substrate with ultrasonic or sealed borders) give a very low baseline of less than 10 μS/cm (which will therefore be the most sensitive quantification for this analysis)
4. Rinsing agent
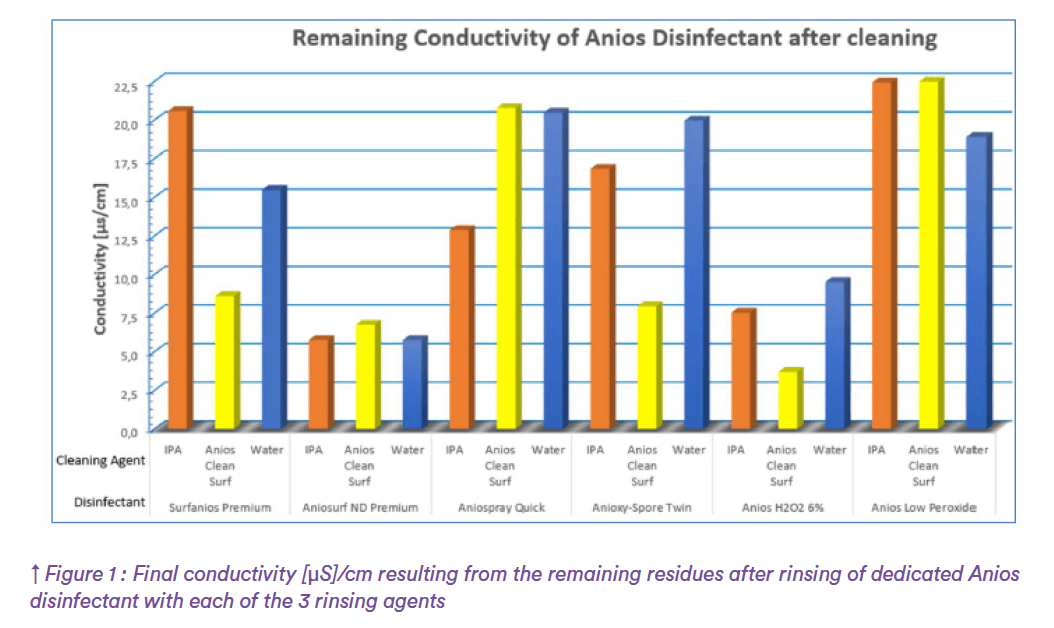
Based on internal study to define the optimal rinsing agent for Anios products (TR AniosCC001)4 it was concluded that on average water removes significantly more residue than Anios Alcool Isopropylique 70% (see Figure 1).
Water is a better rinsing agent due to its ability to easily dissolve ionic or charged molecules. This decision is dependent on the cleanroom grade, especially as it may not be desirable for water to be used as the final product applied in the cleaning and disinfection regime. Given the potential microbiological contamination risks that it may represent. Another effective rinsing agent can then be chosen (IPA for example).
5. Removal technique
Several scenarios were assessed to define the most efficient removal technique and the method described below resulted in a conductivity reduction of almost 90%.
The removal technique is defined as:
1. Apply the disinfectant onto the surface using 100% Polyester Flat Mophead.
2. Leave for the appropriate contact time.
3. Rinse with water.
4. Immediately dry the surface by mopping with a dry mop, ensuring removal of all the remaining liquid.
6. Conclusion
Residues from cleaning and disinfection have received increased regulatory focus and are an important aspect to keep under control. The CCS is a powerful tool to assess and mitigate the residue build up risk.
Conductivity testing is a practical, relevant and easy test method to prove that residue management is well designed in practice. In addition, end-users can switch to “low-residue” product formulations to mitigate and reduce the residue build-up.
Références
*. EudraLex, Volume 4, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1, Manufacture of Sterile Medicinal Products, C(2022) 5938 final (22/08/2022).
*. USP General Chapter <1072>, “Disinfectants and Antiseptics,” USP 42–NF 37 (2019).
* PDA Journal of Pharmaceutical Science and Technology Vol 74 N°2 April 2020. Chapter 9.5 9.5. Residue Removal
* ICH guideline Q9 (R1) on quality risk management, Committee for Human Medicinal Products, EMA/CHMP/ICH/24235/2006 (03/02/2023)
* TR AniosCC001 An Investigation into the Best Rinsing Agent and Process for Overall Residue Management of Anios Cleanroom Disinfectants (September 2023)
Partager l’article

Denis STREITT





