A3P International Congress
Process control and product life cycle: QbD, CPV, Statistical, Analytical / Annex1 - feedback and implementation / Environmental performance
Location
Espace Bellevue, Biarritz (France)
Date
10, 11 & 12 October 2023
Save The Date !

Format
Conferences, workshops, exhibition
Share
Simultaneous translation of the conferences: French <-> English
Tuesday, October 10th
Representatives of the A3P GICs: Qbd & CPV & Analytical & Statistical
Representatives of the A3P CPV GIC
Wednesday, October 11th
The experience of treating women has led Dr. Mukwege to his life's mission: to increase the protection of survivors of sexual violence, and to bring to justice the perpetrators of such violence and those who condone it.
Dr Mukwege is also a member of the Advisory Board of the International Campaign to End Rape and Gender-Based Violence in Conflict. He has received numerous awards, including the 2018 Nobel Peace Prize.
His eco-system consists of a 450-bed hospital, a central pharmacy, a research and training center, a mobile unit and much more... Doctor Mukwege has created a holistic model with 4 pillars, which will be presented to us:
1. Saving lives
2. Repairing souls
2. Gaining independence
3. Demanding justice
A3P Human wishes to highlight his actions, facilitate encounters with the pharma sector and enable everyone to get to know, participate in, donate to, help and support the Panzi Foundation; whether through donations, facilitating access to medicines, care equipment, ... they need us.
https://www.linkedin.com/company/panzi-foundation/
https://fr.wikipedia.org/wiki/Denis_Mukwege
During one day, the participants will exchange, work and debate around a technical or regulatory theme.
Registering for the Congress allows you to participate in one of the 17 workshops that will be presented this year (subject to availability)
Thursday, October 12th
What is a workshop ?
Raise awareness or deepen theoretical or practical notions with the help of case studies that the participants must handle during a workshop in order to propose solutions in the form of conclusions, written with the help of the expert facilitators.
The workshop group is composed of an A3P leader (or moderator) from the Board of Directors, two facilitators chosen for their recognized skills (1 manufacturer and 1 supplier) and attendees or exhibitors who have signed up by name.
Over the course of a day, participants will exchange, work and debate on a technical or regulatory theme.
Registration for the Congress allows you to participate in one of the 17 workshops that will be presented this year ( within the limits of available places). List of workshops below.
- Feedback on the use of the A3P guideline and template by the industry, published in January 2023
- Practical and operational answers to the problems encountered on the sites
- Work on CCS lifecycle management / performance management / Dashboard / KPIs to be developed in the context of continuous improvement
Workshop including a reminder of the APS requirements in Annex 1, feedback from the survey published prior to the conference and work in sub-groups on the TOP 6 of these interpretation and/or implementation issues. The day will conclude with an open roundtable/Q/A session with the expert facilitators.

This workshop is intended for everyone, intermediate to experienced level, and should allow participants to analyze and discuss the critical aspects of the sterile process, to consider various situations of real cases failure that can occur.
Under realistic conditions, the workshop will lead the participants to consider a complete contamination control program, to discover real contamination situations, to search and define together effective CAPA.
Whenever possible, the workshop will illustrate the feedback with the expectation of the new Annex 1.
Objectives :
- How to determine the adequacy of the biocleaning program through the results of the environmental monitoring
- How to ensure that the environmental monitoring program can identify biocleaning failures
- Continued Process Verification (CPV) is part of stage 3 of the lifecycle management of a drug. The objective of this CPV is to provide evidence that the manufacturing process is operating under control state and consistently generates product which meets its predetermined requirements throughout the drug life cycle. This evaluation is done by trending and analyzing data to identify opportunities for process improvement.
- The objective of the workshop "Deployment of the CPV activity for an aseptic repartition workshop" is to realize the deployment of the CPV activity from the identification of the parameters to be followed, the application of the statistical tools and the data analysis, identification and implementation of the corrective actions.
How to identify and control them in relation to continuous process control (CPV)?
- Risk benefits: how to argue for a retro QbD approach? experience feedback
- Setting up retro QbD: What data do I need? How to use it?
- Steps to follow, Pitfalls to avoid
- Continuous Process Verification (CPV)
Working together on the advantages, risks and drawbacks of the retro QbD approach: 1 practical case study in sub-groups
- The aim of the workshop is to consider the various possible options, to select one for each group, and to define the various stages, with their advantages/disadvantages, up to the implementation of the retro QbD.
- The case study will be studied by capitalizing on theoretical and practical knowledge with a concern for balance, cost/benefit, and will be presented to management represented by GIC members..
- Position of scaling in the QBD process
- Case study of an injectable biotech and a dry form
- The challenges of scale-up and the contribution of QbD
For analytical purposes, draft ICH guidelines Q14/Q2(R2) and USP chapter 1220 provide numerous recommendations for an improved approach for a better analytical procedure life cycle management.
But how to set up properly this approach ? With who?
The concrete and tasty approach of this workshop will draw a parallel with daily activities, in order to illustrate clearly the interest of the concept and corresponding tools.
Note: microbiological methods are out of the scope of this workshop
In this workshop, we explore decarbonization opportunities and focus on strategies for implementing sustainable practices while preserving the quality of the end-product.
After a few reminders on the methodology for calculating the carbon balance, we identify, through a practical case, concrete actions that can reduce the carbon footprint and will examine technological innovations such as circular practices and energy efficiency, management of the water...etc.
We will also discuss the economic opportunities associated with the transition to a more sustainable pharmaceutical industry such as reducing energy costs.
The results of this workshop will serve as the basis for developing practical recommendations and strategic roadmaps for decarbonization.
Following a presentation of the general principles that guide the conduct of investigations, participants will study three cases of proven or suspected microbiological non-compliance and the means of investigation available to us to document them in a robust and scientific manner. It will be necessary to define their exact status (proven/unproven), to explain the source, the cause and their impact in order to implement the appropriate corrective and preventive actions where necessary. In particular, we will consider the case of a "non-compliant BioBurden" analysis, a case of a possibly non-compliant PSA and finally a case of a possibly non-compliant sterility test, taken from real industrial situations. The aim will be to identify appropriate strategies for these three types of case and to develop a relevant approach to investigating OOS in microbiology.
On the basis of a shared diagnostic, the workshop will aim to provide practical and pragmatic answers to these difficulties, based on project lifecycle framework when an aseptic production environment system is set up.
Solutions and practices collected during this workshop will enable participants to reuse tips to any other computerized system project implementation.
It will be, indeed, a question of identifying solutions for a control of the physico-chemical and microbiological quality while combining the economic stakes and the strategies for a responsible and sustainable management of pharmaceutical waters.
The participants of this workshop will have the opportunity to share their experience and discuss the problems encountered in the management of their pharmaceutical water installations around a concrete case study.
During this workshop, we will provide practical tools to mobilize teams, communicate about your vision and therefore position yourself as an employer of choice where talents are eager to flourish.
Lean tools, the LEAN mindset and concrete solutions to increase performance - Workshop full
No result without pleasure: this workshop will give you the pleasure to learn and understand TOGETHER why and how LEAN Management will help you improve your expected performance.
To take into account the feedback in terms of improvement from the previous 2 years, the content will be adjusted as follows:
Content
1. Introduction / Round table ==> Sharing among trainees
2. Theoretical contributions illustrated by practical and varied feedback
Feedback from facilitators and participants in terms of REX operational field
3. Implementation via a serious game - experimentation of performance improvements
Technical, organizational, methodological
4. Consolidation of the lessons learned from the exercise via a sequence focused on the state of mind, the methodology, and especially the Lean tools
Make learners aware of the lessons of the exercise
Transpose the keys into a memo (take-away) Specifics of this workshop:
- Serious game
- Contributions from participants prepared in advance
- MMemo containing the keys to effective implementation to use when returning to the company
During this day, four themes will be addressed:
- Air: filtration, clean rooms, air disinfection, clothing
- Liquids: Water production and distribution, filtration and SUU (Single Use System)
- Production equipment: filling lines, isolator and RABS (Restricted Access Barrier System), autoclave
- Quality: laboratory equipment, qualification/validation, services and advice
Full exhibition
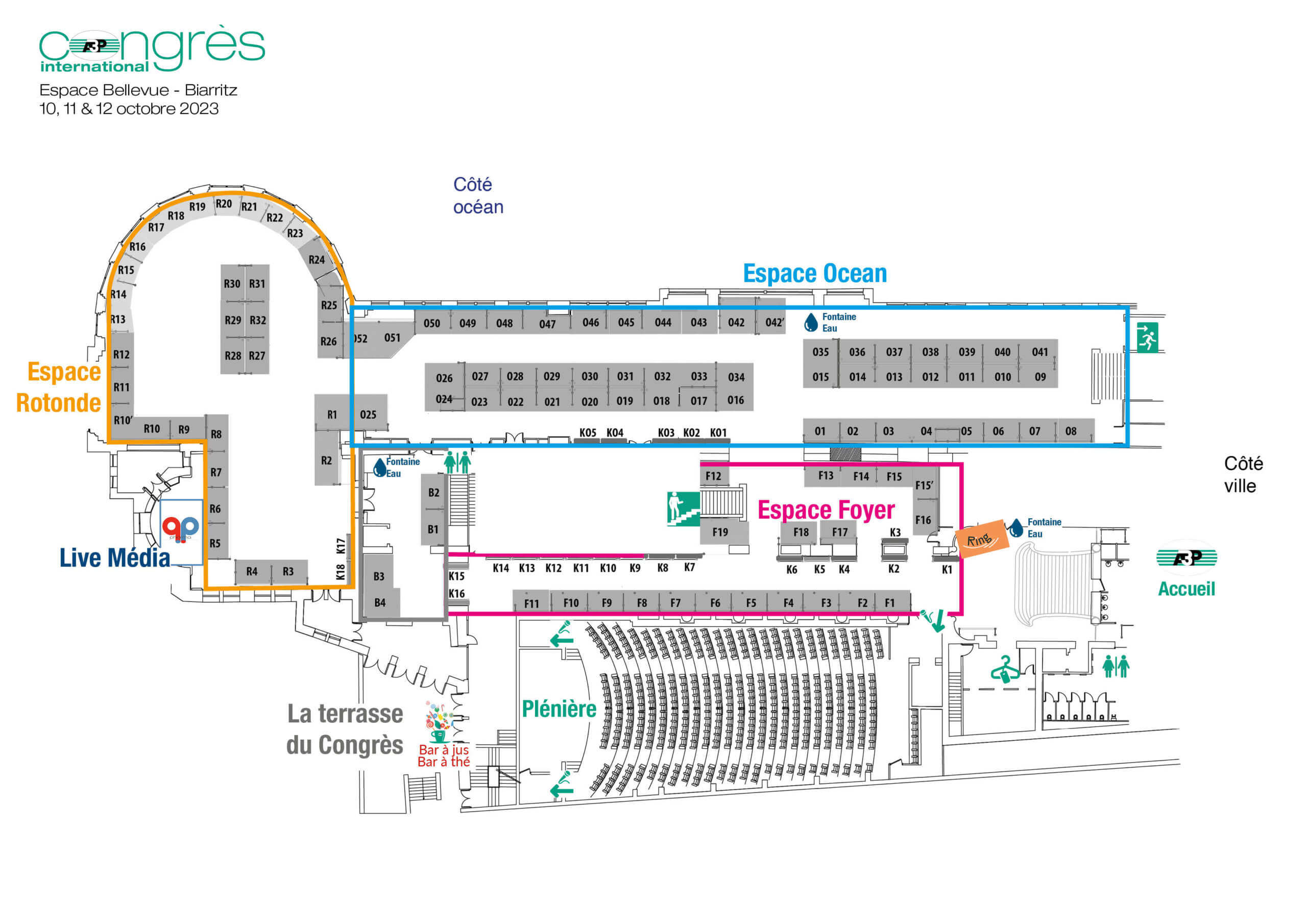
| Société | n°stand |
| ABC TRANSFER | O14 |
| AEROMETRIK | O6 |
| AKTEHOM | F19 |
| ALBHADES | R10BIS |
| AMSONIC – HAMO | R23 |
| ASSOCIATES OF CAPE COD | O36/O37 |
| ATRYON | O13 |
| AVN | K5 |
| BACCINEX | F6 |
| BATIMPRO CHARRIER | R12 |
| BAUSCH+STRÖBEL SE+CO. KG | R7 |
| BD | K16 |
| BECKMAN COULTER FRANCE | O22 |
| BIOMÉRIEUX | O43/O44 |
| BIOPHARMA TECHNOLOGIE FRANCE | B2 |
| BWT | O42 |
| CARBOGEN AMCIS | O38 |
| CARSO LSEHL | R9 |
| CCIT | K6 |
| CHARGEPOINT TECHNOLOGY | F8 |
| CHARLES RIVER | R26 |
| CHRISTEYNS | F4 |
| CONFORMAT | O2 |
| CONTEC | O9 |
| COPHACLEAN | O48 |
| CYTIVA | PALL | O16/O34 |
| DEVEA | R22 |
| ECOLAB | O18/O32/O33 |
| ELIS CLEANROOM | O24/O26 |
| ELLAB FRANCE | K4 |
| ENDRESS+HAUSER | R30 |
| EREA PHARMA | R29 |
| EUROFINS BIOPHARMA SERVICES | F15/F15BIS |
| FPS PHARMA | R15 |
| GASPOROX | R21 |
| GETINGE FRANCE | O10 |
| GIVE & TECH | O3/O4/O5 |
| GOMETROLOGIE | K14 |
| GROUPE ICARE | O11 |
| GROUPE PRODUCTLIFE | K10 |
| HEX + SAFYR | F1 |
| HOF SONDERANLAGENBAU | O50 |
| HONEYWELL – HPS | K17 |
| ILC DOVER | F10 |
| IMA FRANCE | F16 |
| INITIAL | O30 |
| INTERSCIENCE | F12 |
| INTERTEK FRANCE | O27 |
| IWT PHARMA | O42BIS |
| JBT BOURSIER | O21 |
| JCE BIOTECHNOLOGY | O51/O52 |
| KAYE / AMPHENOL | KO1 |
| KHUNER SHAKER | KO3 |
| KÖRBER PHARMA | O19 |
| LABORATOIRE HUCKERT’S INTERNATIONAL | R10 |
| LAPORTE EURO | R27 |
| LIVES INTERNATIONAL | F7 |
| LONZA COLOGNE | O45 |
| LSB – LA SALLE BLANCHE | R32 |
| LUCISBIO | O12 |
| MARCHESINI GROUP | B1 |
| MERCK | F13/F14 |
| MESALABS | R4 |
| MGA TECHNOLOGIES | O28 |
| NEOCERAM | K8 |
| NEOVIX BIOSCIENCES | K2 |
| NOVATEK INTERNATIONAL | R20 |
| OPTIMA PHARMA | O46 |
| ORION LIFE SCIENCES | KO4 |
| OXY’PHARM – SANIVAP | R25 |
| PARKER HANNIFIN FRANCE | R5 |
| PARTICLE MEASURING SYSTEMS | K18 |
| PEMFLOW | K9 |
| PFEIFFER VACUUM | O7 |
| PHARMAPLAN | R24 |
| PHARMASEP | O47 |
| PHARMTEC | O17 |
| PMT FRANCE | O23 |
| PQE FRANCE | KO5 |
| PROSYS GROUP | B4 |
| QESSE by NORDTEST | K15 |
| RAUMEDIC | K7 |
| REALCO | R14 |
| ROMACO FRANCE | F11 |
| ROMMELAG | R8 |
| RT2i | K1 |
| SALAMANDERU | F9 |
| SARTORIUS | O1 |
| SCHOTT PHARMA | O20 |
| SCHREINER MEDIPHARM | O49 |
| SCHÜLKE FRANCE | O29 |
| SGD PHARMA | K12 |
| SGS HEALTH SCIENCE | R28 |
| SHERPAPHARMA | K3 |
| SIDJI | O8 |
| SKAN | F3 |
| SOFAST | F2 |
| SOLIDFOG TECHNOLOGIES | R31 |
| SPC GROUP | K11 |
| STÄUBLI | O40 |
| STAXS | KO2 |
| STERILINE | O41 |
| STERIS | R16/R17/R18/R19 |
| SWAN | R3 |
| SYMBIOSE ENVIRONNEMENT | F17/F18 |
| SYNEXIN | O25/R1 |
| SYNTEGON TECHNOLOGY | O39 |
| TECHNIP ENERGIES | R6 |
| TECHNOCHIM | R13 |
| TEG | B3 |
| TELSTAR | O31 |
| TERANGA GROUPE | O15/O35 |
| THERAXEL | R11 |
| VALTRIA | F5 |
| VEOLIA WATER TECHNOLOGIES | R2 |
| WARANET SOLUTIONS | K13 |
| WILCO | R7. |
Registration form
A3P International Congress
Once you have completed this form, you will receive a copy by email
Registration fee : :
Until June 15: 1200€ excl. VAT (VAT20%) + A3P Membership required (additional costs to be expected if you are not yet a member).
From June 16 to September 12: : 1300€ excl. VAT (VAT 20%) + A3P Membership required (additional costs to be expected if you are not yet a member).
From 13 September: 1430€ excl. VAT (VAT 20%) + A3P Membership required (additional costs to be expected if you are not yet a member).
To request a quote, please complete the form and select the “quote” option in the payment information section
To know if you are a member, please contact us.
To know the rates, please refer to the Membership section of the website: https://www.a3p.org/en/membership/
Contact
Depending on your requirements and preferences, you can contact Ludivine BAYLE (exhibition) or Natalina SEMEDO (program & registration):
Phone : +33 (0)4 37 28 30 40
Email : lbayle@a3pservices.com – nsemedo@a3pservices.com
Accessibility


