Summary
- Quality Assurance & Production: impacts of the new GMP Annex 1.
- Should a visual inspection expert also be a vision system technical expert?
- Residual level of hydrogen peroxide present in isolators during sterility tests: What is the impact on the data generated?
- Advanced Therapy Medicinal Products based on bacteria.
- Supplier & End-User Disinfectant Qualification Comparison for Cleanrooms
- Feedback on the purchase of BFS fillers for a site specializing in PFS.
Advanced Therapy Medicinal Products based on bacteria. Eliminating oxygen from pharmaceutical isolators allows access to new therapeutic fields.
Today we will be focusing on the composition of the atmosphere inside pharmaceutical isolators. This is dependent on the use that is made of them, on internal processes, and to a large extent on the potential for reactivity of the active principles or microorganisms that must be manipulated in the chamber. For years isolators operated mainly under air, under positive pressure or negative pressure, in accordance with their priority use.
Control of humidity and oxygen in the atmosphere of containment chambers has already been perfectly mastered for decades in Research and Industry sectors. Biological laboratories carry out in-depth studies on metalloproteins in glove boxes under anaerobic conditions: crystallization, purification and characterization in particular. Chemistry and physics use glove boxes/ isolators under a pure atmosphere < 1 ppm O2 in the synthesis of products that are ultra-sensitive to the air and the development of new functional materials. By way of an example, glove boxes under an atmosphere controlled for oxygen and/or humidity are enabling extraordinary daily advances in cutting edge fields, such as energy conversion and storage (batteries, photovoltaic cells, OLED…) and securing robotic industrial processes in additive manufacturing and in the nuclear sector. We can therefore state that the technique for oxygen removal is tested and reliable today.
The pharmaceutical sector has been taking a new direction in the monitoring of oxygen for some time. Knowing how to control your atmosphere, particularly the oxygen content, presents undeniable advantages, and is increasingly interesting manufacturers in the pharmaceutical sector in the design of their isolator lines. A choice motivated above all by the fact that new therapeutic fields are seeing the light of day.
We begin with a brief reminder about the control of humidity and the control of oxygen. The techniques used to control humidity and oxygen in the atmosphere of an environment are totally different. The values required also diverge as the objectives are not the same. In addition to their initial function of protection, some pharmaceutical isolators are regularly equipped with humidity control: an atmosphere set to a few percent relative humidity avoids degradation of compounds used. A well-designed purging system allows effective and precise dilution of the internal atmosphere with clean pure nitrogen to work in a stable manner at < 2% RH.
Control of oxygen is more complex as it requires continuous gas treatment, without which the oxygen level rises irremediably, due in large part to the significant permeation of oxygen through materials, gloves and gauntlets in particular. There are in fact two classic methods intended to drastically lower or eliminate the residual level of oxygen in a chamber: the first consists in purging the isolator with a neutral gas (open or semi-open circuit), the second uses a gas treatment scrubber (closed circuit). First and foremost, the manufacturer or the pharmaceutical laboratory must know the “low oxygen threshold” to be achieved when drafting the specifications for the new equipment. This low oxygen level, according to need, is classified according to two major requirement categories: threshold as a % and threshold in ppm.
1. For a requirement level as a % O2
There is an old, simple technique called flushing: via continuous purging with a pure neutral gas (nitrogen or argon), it allows the reproduction of a low oxygen atmosphere which will be suitable for the growth of microaerophilic bacteria, for example. By inerting, the air oxygen level of 20.95 % can be easily lowered to a value of between 2 and 10% O2. This method has an advantage at first sight: its low cost. Nevertheless, this may quickly prove illusory. In fact, although purging with gas appears simple and economic, on use it proves fairly rapidly costly in terms of gas. Improving the purity of the isolator with regard to residual oxygen necessarily goes together with an increased hourly renewal rate and therefore an increase in the flow of incoming gas. Moreover, a desired threshold as a percentage often leaves little room for interrogating the required leak tightness level. An observation that does not take on major importance for isolators operating under air, without control of oxygen or humidity. These incidentally assume their role perfectly by working at a lower positive pressure than that recommended in controlled atmosphere chambers. On the other hand, for chambers working under a modified atmosphere where control of residual oxygen is important, slightly higher working pressures and an increase in leak tightness class to level 1 or 2 in line with the standard ISO 10648-2 are recommended. Failing which, the risk for this type of chamber is that of making oxygen control unreliable, with no possible development towards a future improvement in atmosphere quality.
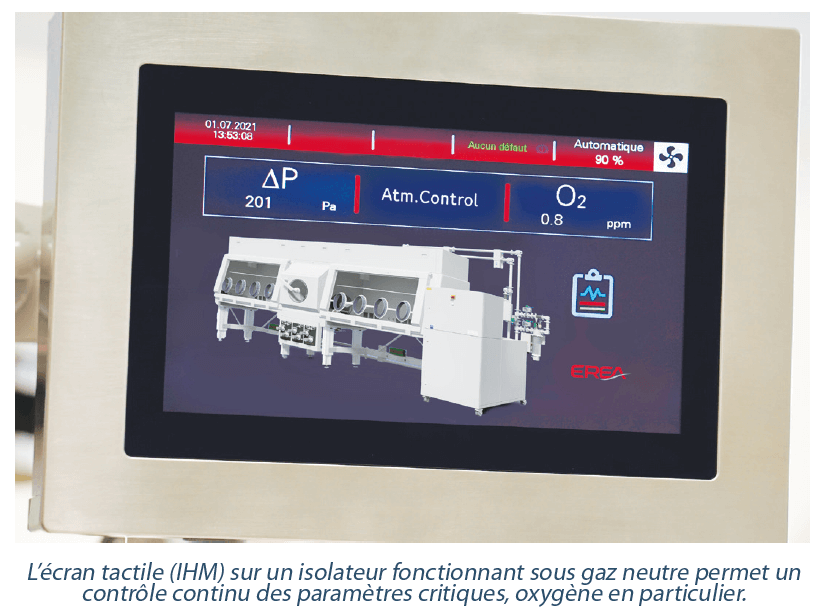
For a low oxygen content isolator project, the specifications are of course defined in relation to purity criteria, but for all that we should not try to demand overly high quality if it is not essential. Thus, for R&D cases that necessitate moderate use of the installation, a percentage O2 test may be appropriate and give good results. For use that is a little more intensive, while remaining in a percentage range, isolators under “intelligent flushing” represent an interesting alternative, a little more economic on gas in comparison with an open circuit. In fact, it is enough to configure your setpoint value as a % O2 on the interface (tactile screen) and a neutral gas circuit will purge the isolator of its air as long as the residual oxygen concentration is too high. When the atmosphere reaches the desired quality, the gas supply is shut off automatically and the isolator then operates on a closed loop on HEPA filters. The system will maintain the chamber at this setpoint and from time to time reinject gas into the circuit in the event that the proportion of oxygen increases.
2. For a requirement level in ppm (parts per million) of O2
Ppm is the fraction equal to 10-6; to better appreciate the oxygen requirement level in ppm, it is enough to perform the conversion: 1ppm O2 = 0.0001% O2. For a test of the atmosphere of an isolator using values in ppm, the gas purging method is to be discounted immediately and only the closed circuit recirculation method can be envisaged.
Pharmaceutical sector manufacturers should consider two aspects in meeting the imperatives of excellent gas purity. On the one hand, as already mentioned, the tightness of the isolator with leak tightness class 1 validation following the standard ISO 10648-2. On the other hand, the connection of the isolator to an effective oxygen removal system, whose characteristics and functionalities should be detailed and clearly fixed to coincide with internal process requirements. These units are among the rare systems currently in place on the market that not only remove oxygen from the working gas in a reliable and lasting manner, but also create reproducible atmospheric conditions.
A neutral gas purification unit allows the gas of the isolator atmosphere to be recirculated in a closed loop on a regenerable scrubber with a catalyst that removes oxygen. This system is fully integrated into an autonomous independent unit named a “gas purification unit” or “gas purifier”, and is connected to the isolator and requires appropriate transfer systems so as to protect the chamber from any atmospheric pollution. A neutral gas purification unit continuously removes traces of oxygen and, depending on the internal process, number of gloves, working pressure (positive or negative pressure), chamber volume and working conditions, it obtains an atmosphere that is stable in oxygen at a residual level of < 5 ppm O2 (commonly within the range 1-10 ppm O2). If needed, it is absolutely possible to supplement the removal of O2 with the removal of H2O at similar levels.
3. Utility of this new form of production space for laboratories
New studies are being conducted by laboratories on a wide variety of stem cells, including anaerobic strains that are extremely oxygen-sensitive (EOS), biological agents and pathogenic microorganisms, GMO and virulent strains…
Controlled atmosphere isolators retain their primary “isolation” function and already provide effective protection for the environment and operators from risks of contact with microorganisms.
Isolators purified of oxygen allow the reproduction of environments comparable to those of the different human microbiota and favor the growth of microorganisms and bacteria specific to each of these. This equipment constitutes cell banks. These are secured reliable experimental working volumes. These anaerobic environments also allow evaluation of the safety of strains, the manufacture of medicinal substances and medicinal products destined to be used in preclinical studies and clinical trials. Validation will finally give the green light to a future commercial supply within a GMP production system.
Purified isolators are also necessary to analytic development, they allow the optimization of tests for the characterization and release of live biotherapeutic products.
4. Why is oxygen toxic ?
Oxygen is toxic to obligate anaerobic bacteria that are extremely oxygen-sensitive (EOS) as they do not have defense mechanisms to protect enzymes from oxidizing agents. Anaerobic bacteria can only multiply on sites with low redox potential. Oxygen may also react with other chemical elements and form specific destructive molecules, peroxides. These peroxides are in particular: the superoxide ion, the hydrogen peroxide ion (H2O2). These are powerful oxidants that destroy organic molecules (DNA, proteins) and that must be eliminated by certain enzymes. Living beings that do not possess these enzymes die in the presence of oxygen and are therefore strict anaerobes.
5. What are the requirements associated with the oxygen removal process ?
The relative speed of the process. The time taken to reduce the atmosphere of the isolator to extremely low oxygen levels, in the order of several ppm, may vary noticeably from one isolator to another. Depending on the size of the chamber and other critical factors already cited previously, it will take several hours to several days of operation of the purification unit to reach the set values.
Prerequisites of the process. Before an oxygen scrubber is brought into operation, the isolator must be previously conditioned. This means that it is necessary to carry out a complete purge of the isolator with a neutral gas for several hours. The copper catalysts used in the oxygen removal process are chemical species intended to purify neutral gases, not the ambient air.
Vigilance to maintain purity. Once the atmosphere is stabilized at a few ppm, any operation, maneuver or manipulation should be considered having regard to the constraints associated with oxygen. All transfer systems (airlock, ports, BIBOs) must be specially designed to avoid any risk of air pollution during their operation and fitted with safety locking devices. The opening of drop-down panels, common on pharmaceutical isolators, must be subject to a prior strict procedure. After the opening of a panel, and ventilation, the reconditioning of the working space with a return to low oxygen values will require an immobilization phase of the installation.
Technical expertise to obtain an ambience < 5 ppm O2. Obtaining an ultra-pure atmosphere of this order is a process that few suppliers in the pharmaceutical world really master. Controlling the oxygen content of the atmosphere of an isolator demands on the one hand use of a perfect purification unit, that is with properly calculated purification capacities and an ideally dimensioned recirculation flow that allows an effective renewal rate. On the other hand, it is imperative that the isolator is validated for leak tightness class 1 in accordance with standard ISO 10648-2, as already stated. This implies that the leak tightness of all components and of the process circuit must also be tested individually.
Regeneration of the purification system. Current purification systems can be compared to sponges. They have a limited adsorption capacity. Once saturated, the scrubber must be regenerated, at the risk otherwise of seeing oxygen levels rise rapidly until the validation of ongoing operations or experiments is called into question. The regeneration of a scrubber, although managed completely autonomously by a robot and an ergonomic HMI, is a tricky operation which involves immobilization of the isolator for hours and makes use of a cycle that combines heating and gas with a low H2 content. This maintenance operation, perfectly programmed by the supplier, should only be envisaged occasionally, which will make the appeal of a purification system all the greater. In fact, this must be justified by a significant saving of gas and act over time so as to procure stable internal purity. The regeneration of scrubbing systems is a long-standing, tested and reliable process. Its circuit is independent of the isolator and does not generate particulate, molecular or chemical transformation of the atmosphere or internal microorganisms. Regeneration is integral and can be performed as many times as necessary for a modest cost and controlled for years.
On the fringes of these extremely effective neutral gas purification systems, but with operating and maintenance conditions that may appear constraining, some manufacturers are trying to develop new alternative processes. Among these new processes, still often at the experimental stage, can be cited, by way of example, those being studied by the Gloveboxes Group:
- the development of new materials derived from the design of nanomaterials or current nanomaterial structures that trap oxygen as well as water.
- the study of new processes using materials with physical-chemical properties that react in contact with oxygen or humidity. Magnetic fields that direct oxygen and water molecules to technical containers.
Behind these studies lies a passion for science and a certain economic value with a stated will to simplify and optimize the oxygen and humidity removal process at industrial level. Despite this, the fact remains that current gas purification processes still have plenty of life in them. In fact, although the principles of the adsorption of humidity and oxygen are old and were developed more than sixty years ago, their performance has improved markedly over time and their properties have been significantly consolidated.
This improvement is partly due to different factors: the industrialization of purification systems supported by a constantly growing market, a design rationalized by means of extremely powerful simulation tools, an improvement in quality and innovative manufacture through ultra-effective production tools offering greater flexibility in construction. The historic manufacturers that developed these systems receive incredible feedback in varied fields which advances them technologically and gives free rein to innovation.
Conclusion
In consequence, the current technologies offered by neutral gas scrubbers are very effective for many reasons:
- their affordable cost with a rapid return on investment in light of performances and results,
- their moderate energy and electrical consumption,
- their infrequent and fully automated maintenance,
- their unequalled capacity to descend to very low levels (ppm) for optimal atmospheric purity,
- their ability to operate continuously 24/7, reliably and durably,
- their potential for recirculation allowing gas to be treated in working and production volumes of several dozen m3,
- their diversity in standard or multi-line systems adapted to all research and industrial production steps,
- their configurable and ergonomic automated management which simplifies operations and reinforces process safety,
- their installations are fitted with ever more reliable sensors and analyzers.
The growing understanding of drug interactions and scientific progress in human biology and physiology have caused the development of advanced therapy medicines for example nanoparticle-based anticancer drugs and of microbiome science. It is certain that the coming years will see great discoveries in these fields. As new therapeutic fields see the light of day, new processes and appropriate equipment will be offered to manufacturers in the pharmaceutical sector. This innovation will allow patients to access ever more effective therapies and ever better targeted medicinal treatments.
Share article

Glossary
Glove box : protective hermetic chamber for manipulations in the nuclear, industrial and research sectors (chemistry, physics, biology).
Isolator : protective hermetic chamber used in the pharmaceutical sector.
ppm : parts per million (10-6); air contains 20.95 % O2, that is 209,500 ppm O2.
Overpressure : positive pressure applied to chambers relative to the surrounding atmospheric pressure.
Depression : negative pressure applied to chambers relative to the surrounding atmospheric pressure.
Open circuit : the air or incoming gas is entirely evacuated to an extraction duct after passing through the chamber.
Closed circuit : the incoming gas is continually and totally recycled, treated and reinjected into the chamber.
Gas purification unit : autonomous system comprising in part a circulator, an oxygen scrubber, an oxygen sensor, valves, automated elements, different gas circuits and a control interface (for detailed explanations, see for example the Jacomex CORE range of purification units).