Summary
- Viewpoint of the ANSM’s Inspection Division (DI) concerning the ICH Q12 guideline
- ICH Q12: the basics. Review of the work of the A3P ICH Q12 GIC
- First steps towards ICH Q12: Leveraging process understanding & development data to define process Established Conditions
- ICHQ12 Implementation from an Industry Perspective with a Focus on Established Conditions
- ICH Q12 compliance and Unified Quality and Regulatory Information Management
- Burkholderia cepacia strikes again
- New EMA Sterilization guideline Guideline on the sterilisation of the medical product, active substance, excipient and primary container (EMA/CHMP/CVMP/QWP/850374/2015)
- How to store highly sensitive drugs? The benefit of functional coatings
Burkholderia cepacia strikes again
The microbiological quality of medicinal products and medical devices is the responsibility of manufacturers. Obviously, no contamination is tolerated in products for which sterility is mandatory and drastic control (quantitative and qualitative) of microorganisms present in non-sterile products is required (absence of specified microorganisms and “objectionable” microorganisms).
In the case of multiple-use products, the presence of preservatives should protect against microbiological contamination during successive uses. The role of preservatives is not to decontaminate a product that does not initially comply, but to maintain a stable microbiological status.
This microbiological quality is guaranteed by the implementation of pharmaceutical quality assurance systems, including Contamination Control Strategy (GMP Annex 1), Quality by Design, personnel training and qualification, the design and qualification of premises, the design and qualification of equipment, requirements concerning utilities (water, air, gas, vacuum), environmental monitoring, the use of active substances and starting materials, including packaging materials in which biocontamination is controlled, cleaning and disinfection of production areas and equipment, the use of terminal sterilization, etc.
All these systems are designed to minimize the risk of contamination of pharmaceutical products and hence to guarantee patient safety. In the context of environmental control, surface disinfection, with the rotation of disinfectant classes, contributes to this objective in various fields: production, quality control and hospital sector. Finally, quality control, with all its imperfections, helps to establish the compliance of products for their release.
In a press release dated November 7, 2019, the French national agency for the safety of medicines and healthcare products (ANSM), indicated that “… Surfa’safe premium disinfectant products in their various forms, along with Opaster Anios, must not be used and have been recalled by the manufacturer“. This voluntary recall by the manufacturer was prompted by the discovery of the presence of bacteria identified as Burkholderia cepacia in several batches of Spray Surfa’safe premium (disinfection of surfaces and non-invasive medical devices) and Pseudomonas oryzihabitans in batches of Opaster Anios (disinfection of endoscopes). The water production system used in the manufacture of these products is thought to have been the source of the contamination. There is therefore a potential risk of human infection because most of the bacteria in the Burkholderia cepacia complex (BCC) can cause serious infections in humans, particularly if they are immunocompromised and especially in cystic fibrosis patients, but also sometimes in people without a compromised immune system. These bacteria have a large genome, giving them extensive metabolic capacities, different virulence factors and multiple intrinsic resistances to antibiotics, disinfectants and preservatives. The disinfectants involved in this recall are widely used in the hospital medical sector, in pharmaceutical sectors (production zones, airlocks, washrooms, etc.) and medical devices (disinfection of multiple-use devices), but also in microbiological control labs!
This recall signifies the immediate cessation of use of these products, recovery of all batches currently in use or unused, and quarantining of the products pending implementation of the procedure to return them to the manufacturer. Testing for the presence of any contaminants in batches used to disinfect production zones and control laboratories, with a view to assessing the potential impact on production and tests, should also be envisaged. This also concerns the use history for these products. Finally, this situation means that a disruption in the supply of these products, which may be of short or long duration, must be anticipated and that it is necessary to find alternative formulations, which will need to be validated. The validation of all disinfection products on receipt is also an issue that needs to be raised, even for those not using the incriminated products. More generally, dissemination in environments (air, surfaces) and utilities (water, network, gas circuits, etc.) should also be considered seriously since the impacts could be devastating.
This is a new episode in the long saga of healthcare product contaminations by members of the Burkholderia cepacia complex (BCC), some of which have caused epidemics, with fatal cases in various regions around the world (Garrity et al., 2005; Jimenez et al., 2007; Torbeck et al., 2011). To gain a clearer picture of the risks associated with these Burkholderia cepacia complex bacteria, we will begin by recalling their major characteristics before looking at the methods used to detect, identify and track them.
1. 1. Major characteristics
1.1 Taxonomy
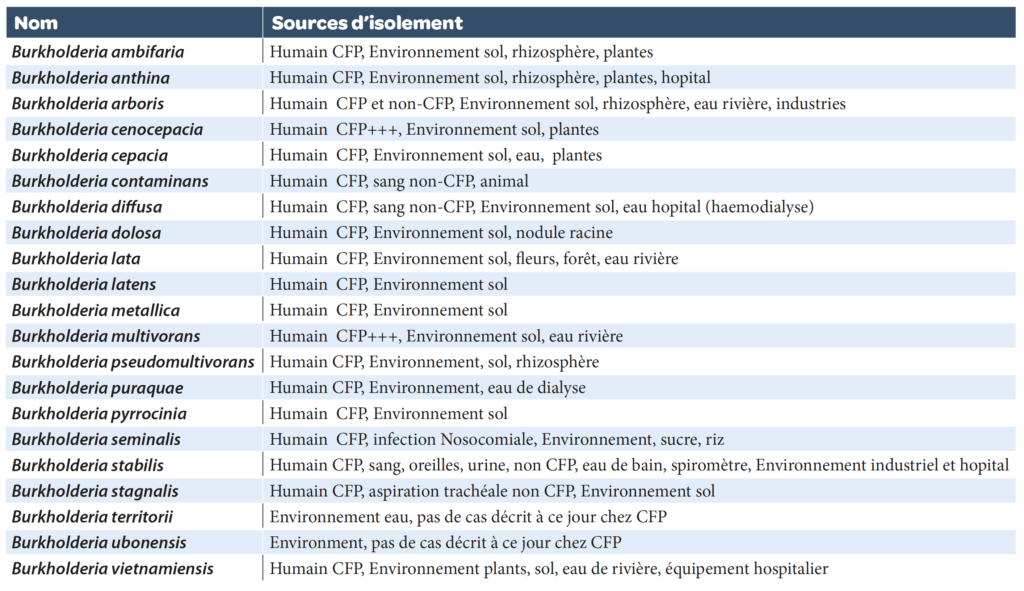
Burkholderia cepacia complex (BCC): the genus belongs to the Burkholderiaceae (Burkholderia, Ralstonia, Cupriavidus, Pandoraea, etc..) family (named after the bacteriologist Burkholder who discovered the agent causing onion skin rot, which was to become known as B. cepacia). They are betaproteobacteria (Gram-negative bacteria) in the form of mobile (polar flagella), aerobic, non-fermenting and non-sporulated, straight or curved rods (0.5-1 x 1.5-4 µm). The type species is Burkholderia cepacia sensu stricto. At present the Burkholderia cepacia complex is composed of 21 validated species that are very difficult to differentiate from one another using usual phenotypic and molecular criteria (Table 1).
1.2 Habitats
These bacteria are some of the most versatile Gram-negative bacteria. They are ubiquitous and are frequently found in natural environments (water, soil, plants [commensal or pathogenic], foods), in industrial environments, including pharmaceutical ones (water systems, air, floor, starting materials, finished products with or without preservatives, saline solutions, disinfectant solutions, etc.) or in hospital environments (dialysis water, water systems). Animals and humans are not considered to be a natural habitat for BCC as knowledge currently stands.
1.3 1.3 Metabolic capacities and dissemination characteristics
Their extensive metabolic capacities, linked to their large genomes (6-9 Mb), mean that they can very efficiently use numerous substrates, even in small quantities (oligotrophic) and colonize environments as variable as pond water, a purified water system, a floor or a plant and its roots, and be an opportunistic pathogen for animals and humans. They also possess numerous genes involved in multiple resistance phenomena to antibiotics, preservatives and disinfectants. They can form biofilms and have numerous virulence factors. They have specific dissemination capacities via water and aerosols, but also from surfaces, where some strains can withstand desiccation conditions for several days in addition to resisting disinfectants. They also have a propensity to be spread from patient to patient (contact, aerosols). They can remain viable in water for several months. Therefore they are very often associated with water and at-risk products are mainly – but not only – aqueous, particularly if these can reach the airways.
1.4 Pathogenicity
BCC species are considered to be opportunistic pathogens. The people at greatest risk are immunocompromised patients (including very young children, the elderly, cancer patients, etc.) and, especially, cystic fibrosis patients (Lipumaetal, 2005; Gautametal, 2016). It is now acknowledged that pathogenic strains in humans can come from the natural environment. Epidemiological studies have demonstrated that the species most often encountered in clinical cases in cystic fibrosis patients are, in decreasing order, B. cenocepacia, B. multivorans et B. cepacia. They present specific tropism for the respiratory system in cystic fibrosis patients. Contamination may be via contaminated aerosols, contaminated disinfectant solutions, contaminated mouthwash solutions, nebulizers, impregnated medical gloves, creams, eye rinse solutions, etc.
Epidemics, including some due to pharmaceutical products contaminated by these species, have been observed in various hospital departments around the world (Australia, UK, Austria, India, Switzerland, USA, etc.) (Marquez et al., 2017). Hyper virulent clones of these species (ST-28 and ST-32 for B. cenocepacia or ST-16 for B. multivorans) have been characterized from several epidemics (Baldwin et al., 2008; Drevinek and Mahenthiranlingam, 2010). Fatal cases have been reported and a significant reduction in the life expectancy of these patients linked to BCC contamination has been demonstrated. Other BCC species only represent around 10% of the clinical cases recorded in humans, but they have all been found, with the exception of B. ubonensis and B. territori. Recent descriptions of these species and precise identification difficulties could explain the absence of documented cases to date. A recent case of a B. stabilis epidemic in a hospital department in Switzerland has been described and documented by whole genome sequencing of isolates (Seth-Smith et al., 2019). The source of the contamination was premoistened gloves used to wash bedridden patients.
A few cases of BCC contamination in non-immunocompromised people have been reported in the literature. Particular attention is paid to very young children and the elderly, who are considered to be at-risk groups. The majority of the species in the complex are encountered in our pharmaceutical environments (A. Carlotti, 2018).
2. BCC and pharmaceutical industries
Burkholderia cepacia complex is one of the contaminants most frequently encountered in sterile and non-sterile pharmaceutical products. It is one of the main causes of pharmaceutical product batch recalls for microbiological reasons.
BCC bacteria are considered to be “objectionable” (undesirable) microorganisms for non-sterile aqueous pharmaceutical products intended for some of the patients at risk from BCC. The sources and causes of BCC contamination of pharmaceutical products have been deduced from experience gained following numerous recalls. These primarily concern: inadequate cleaning procedures, the use of tap water to rinse equipment, inadequate control of water production systems (inadequate disinfection, absence of validation, absence of maintenance, presence of biofilms), inappropriate water system design (formation of biofilms), inadequate microbiological testing, contaminated starting materials (water or other), absence of systematic testing for BCC, use of preservative systems that have not been tested against BCC, inadequate equipment drying procedures, inappropriate storage, inadequate terminal sterilization, inadequate validation of environmental monitoring, etc. These bacteria can develop in solutions containing preservatives; the product is deemed to comply at release, then the initial low level of microbial contamination develops in a few weeks and the product becomes totally contaminated and nonconforming. These sources and causes of contamination should be better controlled.
3. BCC detection, identification and typing
Until recently, there was no official text dedicated to specific testing for BCC bacteria in non-sterile products, in either the USA or Europe. However, due to the increase in cases of pharmaceutical product or medical device contamination since the 2000s, often implicated in hospital-acquired or nosocomial infections in humans, and inherent batch recalls, the FDA has issued numerous warnings about this public health problem and the need for specific controls. In 2018, the US pharmacopeia therefore finally published an urgent (!) preliminary version of a new USP chapter <60> entitled “Microbiogical examination of non-sterile products-Tests for Burkholderia cepacia complex“, for opinions. This text is not harmonized (<1000) with the European Pharmacopoeia. It is applicable from December 2019.
The text recommends using a selective medium for BCC – BCSA (Burkholderia cepacia selective agar) medium – in a standardized protocol in order to determine the absence of BCC in at-risk products, such as products for inhalation, aqueous preparations for oral, oral mucosal, cutaneous or nasal administration. All the bacteria in the BCC described to date are capable of growing on this medium, unlike a large number of other bacteria (e.g. Pseudomonas aeruginosa) which are inhibited by the crystal violet and the antibiotics present in the medium. Some non-BCC bacteria can nonetheless grow on this medium. Suspect colonies on BCSA medium generally appear greenish-brown with a yellowish halo or white with a yellowish-pink halo (phenol red color indicator). This method is presumptive and requires confirmation of the identification of the suspect colonies.
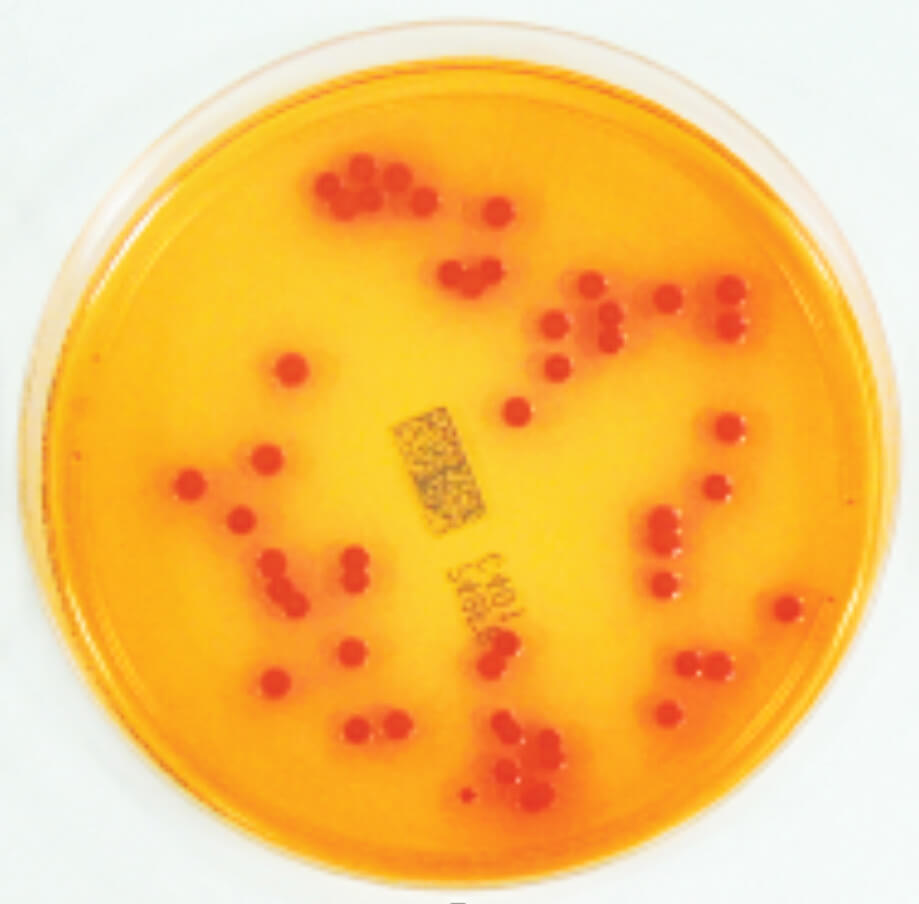
We have transposed this approach to the control of water systems, in which the use of selective chromogenic medium (e.g. Milieu Chrom ID Burkholderia cepacia [CIBC]) (Figure 1) or selective (e.g. milieu BCSA), enables specific detection of members of the Burkholderia cepaciacomplex, mixed with the classic microorganisms of water, much more efficiently than on the usual media not adapted for BCC (A. Carlotti, 2018). Testing for the presence of BCC is sometimes difficult since these bacteria – as is often the case for bacteria in water – do not tolerate a switch from a poor medium to a rich one. These methods are presumptive and all require confirmation of the identification of the suspect colonies.
Testing for BCC bacteria in disinfectant solutions and assessment of preservative efficacy (“challenge tests”) on products are also performed in our laboratories.
Finally, a qPCR test has recently been published for specific testing for Burkholderia cepacia in pharmaceutical products contaminated with these bacteria, even at low levels (Jimenez et al., 2018).
The need for specific rapid methods to detect all BCC members – for in-line/on-line monitoring, for example – for systematic detection is significant.
The identification of BCC complex bacteria is initially sufficient to establish the presence or absence of these undesirable microorganisms in the test conditions.
Precise identification of the species within the complex is essential to assess the magnitude of the threat (B. cenocepaci, B. multivorans and B. cepacia are a much higher risk than B. territori) and to track the contamination pathway or identify the presence of biofilms. In these investigation cases, it may even be necessary to characterize the isolates (typing) within the same species, in order to determine whether the hypothesis of the implication of just one strain in the contamination studied can be retained or not (common source).
Phenotypic identification (biochemical, proteomic) with commercial solutions is difficult for BCC due to the strong phenotypic similarities between species, the variable expression of characters depending on the physiological condition of the cultures and due to the limitations of the solutions available. Mass spectrometry (MALDI-TOF) gives good results for members of the Burkholderia cepacia complex when the species are claimed, irrespective of the system. For example, Fehlberg et al., (2013) reported that 100% of BCC isolates tested were correctly identified with the genus, but 23% were not correctly identified with the species.
We observed good species identification results with the Vitek MS system (A. Carlotti, 2018), with an error of less than 5% for the species claimed in the database for more than 50 pharmaceutical environment isolates assigned to BCC by multilocus sequencing.
Genotypic sequencing with comparative sequencing of genes encoding 16S rRNA (partial [short] or quasi-total [long]) enables reliable identification as a BCC complex member, but not correct identification of the species within the complex since they are not significantly differentiated with this marker. However, we have noted a better performance with quasi-total sequencing compared to partial sequencing for the B. vietnamensis et B. multivorans species (A. Carlotti 2018).
The use of partial unilocus sequencing of the genes recA and/or hisA, generally enables good species identification (Martina et al., 2017). We regularly employ this method for routine tests.
The current method of choice for precise identification of the species is multilocus sequence analysis (MLSA) (Baldwin et al., 2005; Spiker et al., 2009; Martina et al., 2017; A. Carlotti, 2018) of 7 genes (atpD, gltB, gyrB, lepA, phaC and trpB), enabling precise identification of the species within the BCC, but also determination of the molecular type of each strain in the same analysis (typing). We use this method for investigation analyses.
Finally, in our laboratory, we successfully use whole genome sequencing (WGS), which is the ultimate approach for identifying, typing and characterizing the virulence factors and multiple resistances to antibiotics, disinfectants and preservatives of the strains considered. We have obtained very good results for isolates of B. lata and B. contaminans, in particular, from pharmaceutical environments, for which we have obtained an exact identification and typing of strains and identified more than 160 genes involved in virulence and resistance to antibiotics, disinfectants and preservatives (A. Carlotti, 2018; A. Carlotti, 2019).
Conclusion
Burkholderia cepacia complex groups together 21 species of bacteria with extensive metabolic capacities enabling it to colonize numerous environments, particularly water. Some of these species are dangerous opportunistic pathogens for immunocompromised patients (e.g. cystic fibrosis patients) and sometimes for the non-immunocompromised population due to their multiple resistances to antibiotics, disinfectants, preservatives and very unfavorable environmental conditions, their virulence factors and their dissemination capacities. They are some of the most common contaminants of pharmaceutical products (aqueous) and medical devices. Contaminated pharmaceutical products have been a source of sometimes fatal patient contamination or even epidemics in hospital departments on several occasions over the past 20 years.
Current events highlight the importance of this group and the regulatory authorities, via inspections, and batch recalls have encouraged the US Pharmacopeia to finally issue clear directives for systematic testing for the presence of this undesirable microorganisms in pharmaceutical products. We regret the absence of a text of this type in the European Pharmacopoeia, the danger being no less in Europe than it is in the USA. It is the responsibility of manufacturers to assess the risks and take the necessary measures to ensure the microbiological quality of their products. There is a pressing need for specific and rapid microbiological methods for the in-line/on-line monitoring of these microorganisms. However, we have at our disposal all the methods for detection (conventional and molecular microbiology), identification (comparative unilocus, multilocus and genome sequencing) and typing of strains for effective investigations. This also enables pertinent monitoring of pharmaceutical water production systems, validation of disinfectants batch by batch on receipt, and assessment of the efficacy of preservatives targeting BCC. We are actively working on methods to control and prevent this type of contamination.
It is interesting to note that on March 18 and 19, 2020, the A3P Association will hold its Biennial Microbiology Congress in Tours. The program includes four themes: Alternative methods in microbiology, Microbiological testing of biological products, “In-line/on-line” microbiological controls and a section on the lessons to be learned from “warning letters” and injunctions, for microbiological reasons.
We will thus be able to carry out an in-depth analysis of these concrete and topical issues within our professional network.
Share article

Arnaud Carlotti – EUROFINS
For over 30 years, Arnaud CARLOTTI has been passionate about industrial microbiology and production issues in sterile conditions, investigations of non-conformities, root cause analyzes, proposal and implementation of CAPAs.
As a recognized expert, he has published more than 30 scientific articles and book chapters to date (eg. 2018 PDA / DHI publishing “Contamination Control volume 5 chapter 14).
Bibliography
Carlotti A. (2018) Burkholderia cepacia complex several absolutely-objectionable bacteria species. In Contamination control in healthcare product manufacturing, Volume 5, Chapter 14, Madsen R. E. and Moldenhauer J. editors, PDA Bethesda and DHI River Grove Publishing.
Carlotti A. (2019) Bactéries de l’espèce Burkholderia lata dans l’industrie pharmaceutique, apports de la génomique. Quinzième Congrés de la Société Française de Microbiologie, ” Microbes “, 30 septembre au 2 Octobre, 2019, Cité des Sciences et de l’Industrie, Paris.
Baldwin A., Mahenthiralingam K., Thickett D., Honeybourne M., Maiden et al. (2005) Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J Clin Microbiol 43, 4665–4673.
Baldwin A., Mahenthiralingam E., Drevinek P., Vandamme P., Govan J., et al. (2007)
Environmental Burkholderia cepacia complex isolates in human infections. Emerg Infect Dis, 13, 458–61.
Drevinek P. and Mahenthiralingam E. (2010) Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol and Infect Dis, 16, 7, 820–830.
Fehlberg L., Andrade L., Assis D. et al., (2013). Performance of MALTI-Tof Mass Spectometry for species identification of Burkholderia cepacia complex clinical isolates. Diag Microbiol and Infect. Disease 77, 2, 126-128.
Garrity G., Bell J. and Lilburn T. (2005) Burkholderiaceae in Bergey’ manual of systematic bacteriology, second edition, Vol. 2. The Proteobacteria part C. Garrity G. editor. New York, Springer.
Gautam V., Patil L., Kuma S., et al., (2016) Multilocus sequence analysis reveals high genetic diversity in clinical isolates of Burkholderia cepacia complex from India. Sci Rep, 6, 1-9.
Jimenez L. et al. (2007) Microbial diversity in pharmaceutical product recalls and environments. PDA J. Pharm Sci Tech, 61, 5, 383–399.
Jimenez L. et al. (2018) Real-Time PCR Detection of Burkholderia cepacia in Pharmaceutical Products Contaminated with Low Levels of Bacterial Contamination. PDA J Pharm Sci and Technol, 72, 73–80
Lipuma J. (2005) Update on the Burkholderia cepacia complex. Curr Opin Pulm Med, 11, 6, 528–33.
Marquez L., Jones K., Whatley E., Koy T., Revell P. et al. (2017) An outbreak of Burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol, 38, 5, 567–573.
Martina P., Leguizamon M., Prieto C., Sousa A., Montanaro et al. (2018). Burkholderia puraquae sp. nov., a novel species of the Burkholderia cepacia complex isolated from hospital settings and agricultural soils. Int J Syt Evol Microbiol, 68, 14–20.
Seth-Smith H., Casanova C., Sommerstein Rami, et al., (2019) Phenotypic and genomic analyses of Burkholderia stabilis clinical contamination in Switzerland. Emerg Infect Disease, 25, 6, 1084-1092.
Spilker T., Baldwin A., Bumford A., Dowson C., Mahenthiralingam E., and LiPuma, J. (2009). Expanded Multilocus Sequence Typing for Burkholderia Species. J Clin Microbiol, 47, 2607–2610
Torbeck L. Raccasi D., Guilfoyle D., Friedman R., Hussong D. (2011) Burkholderia cepacia : This Decision Is Overdue. PDA J Pharm Sci Technol, 65, 5, 535–543.




