Summary
- 6 Sigma and Operational Excellence. Just Common Sense?
- How many values are necessary to have a representative sample?
- Statistical modeling: The need for a reliable approach to improve process knowledge and understanding
- Bayesian approach in cosmetical research : Application to a meta-analysis on the anti-pigmenting effect of vitamin C
- Comparability, equivalence, similarity… How statistics can help us to demonstrate these. And soon, the end of blind testing for health authorities and manufacturers.
- Maintenance of the validated state, a stage in the validation cycle.
Validation is divided into five major phases which are :
– Planning and definition of needs
– Definition of specifications and configuration
– Verification
– Use
– Decommissioning
The validation process can be illustrated by the life-cycle loop shown opposite.
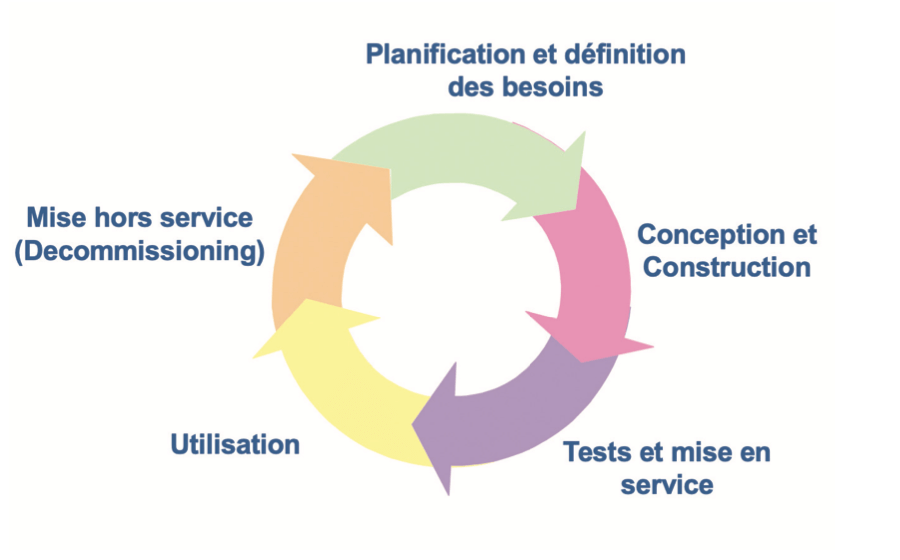
After the definition of needs, design and qualification (tests and commissioning) phases, comes the use phase in which maintenance of validated state should be verified. This step, generally the longest, has the objective of proving that the item in question is maintained continuously in a validated state throughout its use.
What the regulatory texts say
- Eudralex(1), volume 4, Annex 15, paragraph 4 (March 2015) :
“Equipment, facilities, utilities and systems should be evaluated at an appropriate frequency to confirm that they remain in a state of control”. - BPF(2), Annex 15, paragraph 4 :
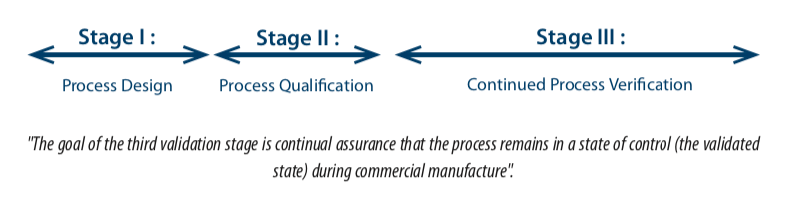
“Equipment, facilities, utilities and systems should be evaluated at an appropriate frequency to confirm that they remain in a state of control”. - The FDA Guidance document(3), Guidance validation : General principles and practices, paragraph 4– January 2011, defines the validation cycle in 3 stages:
In practice
In order to meet the different regulatory requirements and to guarantee the maintenance of the validated state it is necessary to put in place different processes in a robust manner.
• • The procedures for use should be clear and detailed in order to facilitate their understanding by all persons who may use them, and to make reproducibility during performance of the different tasks possible. It is necessary that the critical parameters of the process, defined during the qualification stage, are described and identified in the procedures.
• Each person who performs or supervises drug manufacture operations should undergo the staff training process in place in the company. This process should be clear, detailed and rigorous. In the context of certain critical activities, it is necessary to conduct an accreditation process, such as, for example accreditation in manual visual inspection for individuals who carry out inspections during production, media fill (MFT) accreditation for operations performed in an aseptic environment. These accreditations should be re-evaluated periodically in order to check there has been no deviation.
• The change management system in place should allow the impact of changes made to validated equipment, systems or processes to be controlled. The change management process should describe the measures to be implemented in the event of modification of a raw material, of a product component, of production equipment, of all or part of a computerized system, of the manufacturing environment, of production or test method, or any other change liable to affect product quality or process reproducibility. Change control can be provided via the performance of an impact assessment for each change by a multidisciplinary team (for example regulatory, validation, maintenance, production sectors…).
• For the most critical systems, a periodic qualification is a regulatory requirement in parallel with preventive maintenance (cleaning, filling, visual inspection, sterilization, aseptic filling processes…). Periodic qualification of a system is understood to mean all activities that demonstrate that the critical functionalities as defined during the design phase are always under control. In some cases, periodic qualification can be substituted for certain tests such as:
– the preventive maintenance and calibration activities which include tests of critical functionalities (alarms, ejection systems)
– challenge testing mainly for packaging operations and suitability testing in the control laboratory, to ensure a system is functioning correctly before the start of operations.
Test frequency should be based on a risk analysis in which the results of previous qualifications, equipment or system criticality can be incorporated.
• All facilities, utilities, equipment and computerized systems should be assessed at an appropriate frequency to confirm that they indeed remain under control. This can be tracked through via a periodic validation review. This review should allow :
– provision of proof of the maintenance of the validated state for all elements which directly impact product quality.
– identification of the need to requalify or revalidate all or some of these items.
• Preventive maintenance should be managed and implemented from the time that use of equipment item begins. For this, during the qualification stage, an analysis of the different components of this equipment item should be conducted. On the basis of this analysis, the associated maintenance plans, classified according to criticality, should be put in place. This is in order that equipment is maintained and checked so allowing its validated state to be preserved.
• The periodic product quality reviews should be conducted a set frequency. During this review, the monitoring of critical quality attributes and critical Quality parameters enable product quality and process performance to be tracked so verifying process repeatability and confirming maintenance of the validated state.
In conclusion, control of the initial qualification of equipment and compliance with the previously-cited processes during the use phase are essential in order to then be able to put in place continued process verification (CPV), in such a way as to detect any deviations. This will allow action to be taken before a nonconformance occurs.
Several questions arise with respect to the setting up of CPV. A working group (CIG) is studying this subject in order to create a guide to assist with its introduction in industries that use aseptic filling procedures.
Share Article

Sabrina GALLAY – ASPEN
Expert validation since 12 years (process validation, cleaning validation, article validation). I specialized on maintaining validated status since 4 years. Aspen Notre Dame De Bondeville
sgallay@fr.aspenpharma.com
Bibliography
(1) Eudralex (1), volume 4, Annexe 15, paragraph 4 (mars 2015)
(2) BPF, Annexe 15, paragraphe 4.
(3) Guidance validation : General principles and practices, paragraph 4– January 2011