A3P Microbiology
ARMM, Regulations, Data Integrity, Microbial identification, Bioburden, Subcontracting of microbiological methods, Annex 1
Location
Palais de la Musique et des Congrès Strasbourg (France)

Format
Conferences, Partner sessions, Exhibition
Share
Every two years, the A3P Microbiology days is a major event that brings together analytical microbiology professionals from the pharmaceutical and cosmetics industries.
For the first time, they will be held in Strasbourg on Wednesday June 26 and Thursday June 27, 2024.
True to tradition, this event will address the major opportunities and challenges for the future of analytical microbiology in our industries.
Join professionals from the pharmaceutical sector and regulatory authorities, to share and discuss best practices, regulatory developments and the development, validation, and implementation of innovative, alternative and automated technologies.
The program and structure of this event are based on the exchange and sharing of experience through:
- – 14 oral presentations,
- – Exhibition of key industry suppliers
- – Interactive workshops
The main topics will be:
- – European and American regulations relating to alternative methods and the latest changes and new texts, with the participation of pharmacopoeias representatives.
- – Essential microbial identification methods to comply with the new Annex 1.
- – Alternative methods in microbiology, with successful implementations and the latest innovations to come.
- – An update on automated methods in analytical microbiology.
- – New approaches to assessing bacterial endotoxins and pyrogenicity.
Come all to listen and to interact with all the key players and suppliers in the analytical microbiology field, which remains the fundamental element of our clean and sterile products.
Simultaneous translation: French <-> English
Wednesday 26 June 2024
Thursday 27 June 2024

Wednesday, June 26, 2024
Session 1 – 11h30
- Automatisation des tests d'endotoxines : Présentation d’un robot autonome.
- Futur du LAL et développement durable : Cartouches Trillium compatible avec vos lecteurs actuels : mise en œuvre simplifiée d’une solution durable pour vos dosages d’endotoxines
- Validation d'une méthode recombinante Trillium : approche règlementaire et validation de cette méthode
Session 2 – 14h30
- Les Attentes des inspecteurs, exemple de Warning Letters et rappel produit
- Comment interpréter les résultats et réussir son monitoring environnemental: Outil simplifiée pour l'EMPQ (Environmental Monitoring Performance Qualification) de vos Zones à Atmosphère Contrôlée (salle blanche, isolateurs)
- Démonstration d’un outil de suivi de tendance et Accupedia.
Thursday, June 27, 2024
Session 3 – 10h30
- Les Attentes des inspecteurs, exemple de Warning Letters et rappel produit
- Comment interpréter les résultats et réussir son monitoring environnemental: Outil simplifiée pour l'EMPQ (Environmental Monitoring Performance Qualification) de vos Zones à Atmosphère Contrôlée (salle blanche, isolateurs)
- Démonstration d’un outil de suivi de tendance et Accupedia.
Session 4 – 11h30
- Automatisation des tests d'endotoxines : Présentation d’un robot autonome.
- Futur du LAL et développement durable : Cartouches Trillium compatible avec vos lecteurs actuels : mise en œuvre simplifiée d’une solution durable pour vos dosages d’endotoxines
- Validation d'une méthode recombinante Trillium : approche règlementaire et validation de cette méthode
Full exhibition
Exhibitors
| Company | N° Stand | Company | N° Stand | |
| GETINGE | 1 | EUROFINS BIOPHARMA PRODUCT TESTING FRANCE | 16 | |
| EUROFINS BIOTECH GERMANDE | 2 | HEX + SAFYR | 17 | |
| MICROCOAT BIOTECHNOLOGIE | 3 | SYMBIOSE ENVIRONNEMENT | 17B | |
| JCE BIOTECHNOLOGY | 4 | BIOMÉRIEUX | 18 | |
| STERIS | 5 | BD | 18B | |
| BRUKER FRANCE | 6 | ELSCOLAB | 19 | |
| MERCK | 7 | ECOLAB | 20 | |
| CARSO LSEHL | 8 | ALLIANCE BIO EXPERTISE | 21 | |
| GROUPE ICARE | 9 | MESALABS | 21B | |
| ALBHADES | 10 | CHARLES RIVER | 22 | |
| TERANGA GROUPE | 11 | DEVEA | 23 | |
| PLAIR | 12 | REDBERRY | 24 | |
| INTERSCIENCE | 13 | EREA | 24B | |
| ASSOCIATES OF CAPE COD | 14 | REALCO | 25 | |
| SKAN | 15 | STAXS | 26 | |
| RAPID MICRO BIOSYSTEMS | 15B |
Exhibition plan
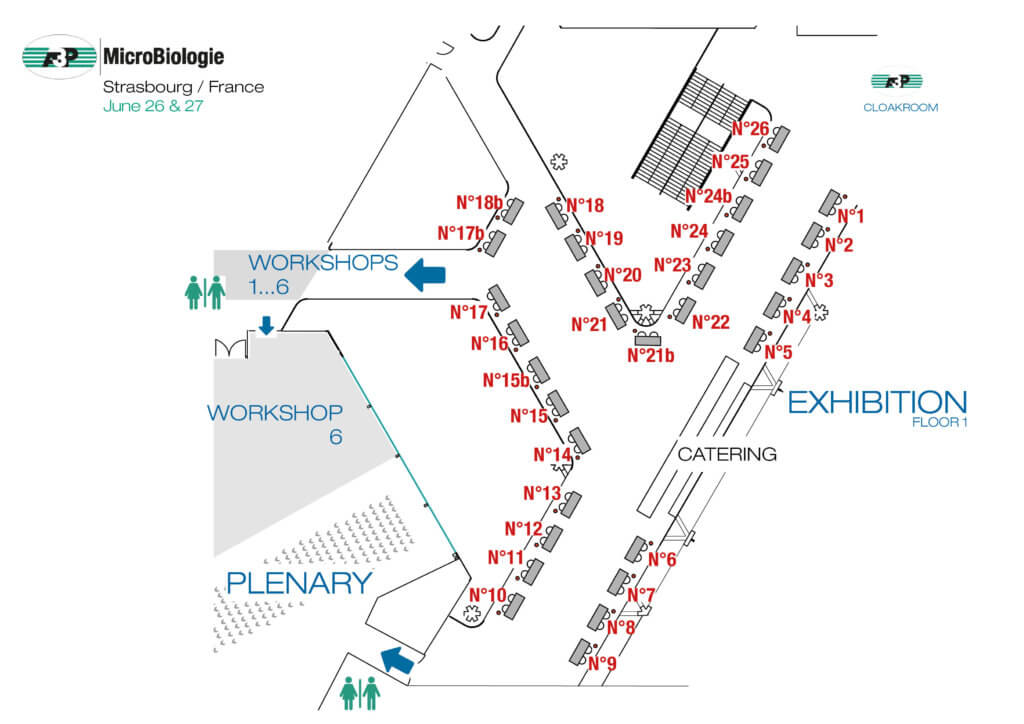
*Exhibition layout subject to change
Services included with the Table Top stand
- A dedicated exhibition space where you can set up your products and communication tools: umbrella stand, roll-up, display, products, etc. Basic equipment including a 140×70 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker, and the loan of a Nespresso coffee machine with pods.
- Breaks and meals as indicated in the program
- Unlimited WiFi
- A badge giving you free access to the entire technical and scientific program
- The list of event participants > available on the A3P mobile application
- Possibility of registering an additional person at the preferential “accompanying person” rate (€900 excl. VAT)
Registration fee: €1,200 excluding VAT + compulsory A3P membership (additional costs apply if you are not yet a member).
To find out if you are a member, please contact us.
For prices, please see: https://www.a3p.org/en/membership/
Contact
Depending on your requirements and preferences, please contact:
- Natalina SEMEDO (program & registration) : nsemedo@a3pservices.com / + 33 (0)4 37 28 30 52
- Ludivine BAYLE (exhibition & partners) : lbayle@a3pservices.com / + 33 (0)4 37 28 30 46
Accessibilité



