Summary
- Analytical Quality by Design: the required integration for Quality by Design
- Design of a production isolator. From user need to realization.
- Advanced vaporized H2O2 decontamination technology for pharmaceutical isolators. Reduction of H2O2 decontamination cycle time using direct injection nozzles.
- Secure the containment of your gloves
- Isolator Technology and Automation Enhanced Contamination Control in the Manufacture of Cell and Tissue Culture Derived Regenerative Medicine Products.
- The European approach to disinfectant qualification.
There are two types of gloves for isolators and other containment systems:
• so-called ‘one piece’ gloves which extend from the glove port from the shoulder to the finger tips
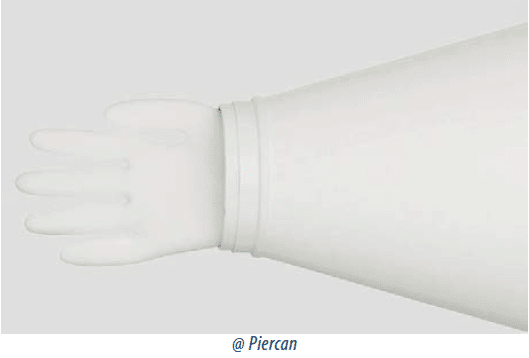
• so-called ‘glove-arm guard’ gloves which consist of an arm-guard extending from the glove port from the shoulder to the cuff ring then a glove extending from this ring to the fingertips.
This last type of assembly allows for glove replacement where a different glove size is needed or in the event of accidental perforation.
Why develop a new connector for the glove and arm guard?
The changing and fixing of gloves to arm guards on the rings that are currently used is problematic as a result of:
• the complexity of the change which carries a risk of loss of containment during manipulation and glove replacement;
• the force required to replace the glove which can cause musculoskeletal problems;
• the small numbers of users authorised to carry out glove replacement;
• the fact that users doubt the efficacy of the systems used;
• the risks of micro-cuts in the glove at the wrist cuff ring
• risks of dislodging the gloves during manipulations.
In order to facilitate the work of operators, a secured connector ring allows:
• glove replacement without the use of tools,
• glove fixation and removal with little effort,
• efficient glove replacement by all users through this intuitive solution,
• glove replacement in 3 minutes maximum,
• that the part of the glove in contact with the coupling is no longer under tension and remains protected by the body of the arm guard coupling avoiding cuts to the glove at the level of the wrist,
• that the mechanical resistance of the glove is not dependent on the variability of the O-ring.
How to replace the glove with the new secured connector ring?
Step 1: tuck the glove inside the arm guard
Step 2: position the 3 clips of the glove coupling, with the 2 arrows at the top facing the grooves of the arm guard coupling
Steps 3 to 4: push the glove in, a click is heard, the glove is locked
Step 5: the glove to be removed is ejected outside the arm guard
What level of performance can be expected from this new secured connector ring?
The connector ring materials are compliant with the FDA requirements (paragraphs 177.1660 and 178.2010 CFR21). In addition, the PBT material of this connector demonstrates excellent biocompatibility on the USP 23 class VI test.
These materials accept sterilisation with hydrogen peroxide vapour, autoclaving and irradiation. They are not degraded by isopropyl alcohol.
The result of the sporicidal D-value measurement of the PBT of this connector is comparable to stainless steel:
• PBT : 1.2 minute ;
• Stainless steel: 0.9 minute.
Piercan has qualification documents for particulate decontamination according to the standard of the class 200 IEST STD CC 1246D and for sterilisation by gamma radiation according to the VDmax25 method of standards NF ISO 11137 and 11737.
The arm guard-glove unit mounted on this connector complies with the requirements of the standard EN 421. The tensile strength of the glove on the arm guard is greater than 400 N.
The performance levels of the gloves mounted on this new connector according to standards EN 420, EN 374 and EN 388 are preserved.
The bacterial tests demonstrate the efficacy of the leaktightness of this secured connector in use and during glove replacement.
During manipulations, the protection of the glove at the wrist enables cuts to the glove close to the bead to be avoided.
In addition, the endurance tests carried out confirm a possible reuse of the glove mounted on its connector up to 20 times.
Glove replacement can be carried out easily by all users in a shortened time of 3 minutes.
The following materials are available:
Neoprene, CSM, CSM/PUR/CSM or EPDM for the gloves, and PVC, Neoprene, CSM or EPDM for the arm guards.
The secured connector functions in positive pressure isolators (gloves ejected outside the isolator) and negative pressure isolators (gloves ejected inside the isolator).
Conclusion
The use of this type of glove during production and/or tests in the biopharmaceutical field allows glove size and thickness to be adjusted appropriately in accordance with staff and workstation.

Franck ARETHUSE – PIERCAN
franck.arethuse@piercan.fr
Share article
Glossary
CSM : Chlorosulphonated polyethylene (formerly Hypalon ®).
D-Value : Time required to eliminate 90% of bacteria.
EN 374 : Gloves for protection against chemicals and micro-organisms.
EN 388 : Gloves for protection against mechanical risks.
EN 420 : General criteria relating to protective gloves.
EN 421 : Gloves for protection against ionising radiation and radioactive contamination.
EPDM : Ethylene propylene diene monomer.
F.D.A. : Food and Drug Administration.
PBT : Polybutylene terephthalate.
PUR : Polyurethane.
Bibliography
USP 23 Class VI : United States Pharmacopeia
Classe 200 IEST STD CC 1246D : Product cleanliness levels – Applications, requirements and determination.
Standards NF ISO 11137 and 11737 : Sterilisation of health care products and medical devices.
Standard EN 421 : Gloves for protection against ionising radiation and radioactive contamination.
Standard EN 420 : Protective gloves – General requirements and test methods.
Standard EN 374 : Gloves for protection against chemicals and micro-organisms.
Standard EN 388 : Gloves for protection against mechanical risks.
Bacterial tests : Protocol developed by PIERCAN.