Summary
- Some good validation practices for analytical procedures
- ALCOA …with an A for Accuracy
- Established Conditions for Analytical Procedures and QbD: current situation and future perspectives for enhanced change management paradigm
- When the vibrations of molecules make their assay possible: near infrared spectroscopy in action
- Input from accelerated predictive stability (APS) studies to pharmaceutical development
- Implementation of green analytical chemistry in the quality control laboratory of the company UPSA
- Evaporation of alcoholic solutions. What residues are left on equipment?
Evaporation of alcoholic solutions. What residues are left on equipment?
lt is common not to look for potential residues of alcoholic products as it is understood by all that these volatile products do not leave any residue on equipment after evaporation. The objective of this article is to present the results obtained by a laboratory study which demonstrates this theory scientifically and practically. This study thus provides a scientific argument to justify dispensing with tests for alcohol residues on equipment.

1. Objective and principle of the study
The objective of this study was to demonstrate the presence, or absence, of carbon-containing residues after evaporation of products composed of different types of alcohol, currently used on production equipment in the pharmaceutical and cosmetics industry.
Indeed, the presence of residues of alcohols or additives (identified or not) could potentially contaminate the next production operation on the equipment in question.
This study also checked that the use of these “no rinse” products does not influence TOC analyses in the context of cleaning validation or monitoring, by generating false positive results.
2. Materials
In this study, we studied 3 alcoholic solutions that are representative of products used on the ground: a sterile solution of 70% ethanol, a sterile solution of 70% isopropyl alcohol and pure 99.6% isopropyl alcohol.
The analyses were conducted with a Sievers M9 Portable TOC Analyzer equipped with a sample feeder (Suez). The vials used for sample preparation were treated specifically for TOC meter analyses, certified as having a low TOC content.
Ultrapure water was used for all of the study, the TOC content of which is checked periodically. The preparations were produced using an Eppendorf® Multipette E3X and the associatedcombitips.
3. Operating method
In order to be able to judge the quantity of residual carbon after evaporation, samples of evaporated and non-evaporated alcohols were analyzed in a TOC meter, then the contents obtained for each type of sample were compared.
Apart from product type, it was judged important to check the influence of the concentration of the solution initially present on the quantity of residue found after evaporation. Consequently, for each type of alcoholic solution, 3 concentration levels were deposited so as to obtain results in the order of 10, 30 and 50 ppm TOC after dilution in 40 ml ultrapure water.
In addition, in order to confirm the results obtained, two independent series of tests were carried out on two different days, with a gap of several days. So, for each concentration and for each type of solution studied, two tests were carried out using different preparations.
The tests carried out in duplicate on several concentrations allowed us to check the linearity and the repeatability of the concentrations obtained. We chose to deposit the solutions directly into the final analysis vial in order to protect the samples from contamination due to a transfer of container. The results obtained therefore apply to evaporation of the product deposited on glass.
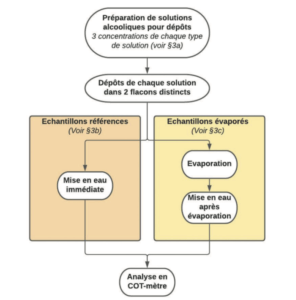
3.1 Preparation of deposit solutions
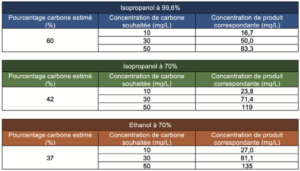
The concentrations of the solutions to be prepared to reach the content cited previously were determined through the carbon percentage calculated theoretically (from the empirical formulae of the different compounds) for each of the solutions:
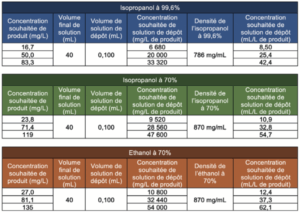
In order to be free of a possible influence of deposit size on the final quantity of residue after evaporation, we chose to standardize the volume deposited for each type of solution and for each concentration studied. Consequently, it was necessary to prepare 3 deposit solutions for each type of alcoholic solution studied (one solution per concentration level).
The theoretical concentrations desired for the deposit solutions were as follows:
In order to avoid any partial evaporation, we chose to select a volume of alcoholic solution rather than carrying out a weighing operation. Volume transfer allows the solution to be exposed for a much shorter time than during a weighing operation. The deposit solutions were thus prepared as described below:
Sample preparation consisted in depositing 100μL of each deposit solution prepared beforehand, then in adding ultrapure water until the final volume of 40mL was reached (immediately after the deposit or after evaporation of the deposit), the corresponding concentrations for the solutions prepared in this way were, consequently, the following:
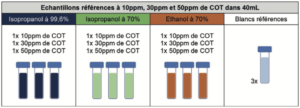
3.1 Preparation of reference samples
These preparations had a dual aim: on the one hand to check that the TOC meter was capable of analyzing and supplying the results projected by the theoretical calculations; and the other hand, to also obtain a reference value for the interpretation of evaporated samples. The quantity of carbon so determined by the TOC meter corresponds to the quantity of carbon present before evaporation. It was used to calculate the efficiency of the evaporation of carbon-containing products.
The reference samples were prepared in several steps:
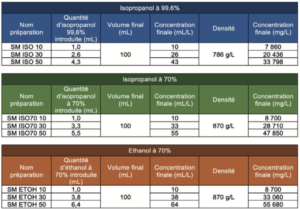
Step 1: Deposition of 100 μL of ethanol or isopropyl alcohol deposit solution prepared beforehand at a concentration that allowed the desired levels to be achieved, directly in a vial for TOC analyses.
Step 2: Immediate addition of 39.9 mL ultrapure water to reach a final volume of 40mL. As the reference samples were used to determine the quantity of carbon present in the quantity of alcoholic solution deposited, water was added to the TOC vials which were then closed, immediately after depositing the product in order to avoid any partial evaporation. The immediate closure of the vials also avoided any partial inter-sample contamination. For the same reason, each sample of each type of alcohol was prepared and processed independently from the others, so as to never have several TOC vials open simultaneously.
The analytical technique used for this study was non-specific (TOC meter). It was therefore also necessary to prepare sample blanks to determine the quantity of carbon contributed by the equipment and the ultrapure water and thus to be able to deduct this value from the different samples.
Step 3: For the preparation of solvent blanks, 40mL of the ultrapure water used in step 2 of the preparation of reference samples was introduced directly into a TOC vial. For each test series, 3 vials of blanks were prepared in order to obtain a more robust TOC content value. The mean of 3 blanks was used to deduct TOC content from the reference samples.
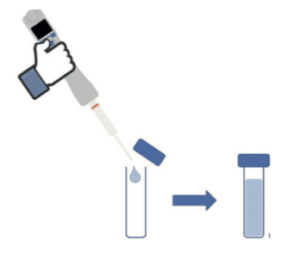
The nature and quantity of each reference sample prepared, per series, are summarized below:
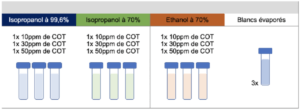
3.3 Preparation of evaporated samples
The evaporated samples were the subject of the study. These were samples of alcoholic solutions left deliberately to evaporate in order to study and detect potential carbon-containing residues, once the surface was dry. They were prepared in a manner strictly identical to reference samples and from the same deposit solutions for each test series.
The preparations for each of the evaporated samples were as follows:
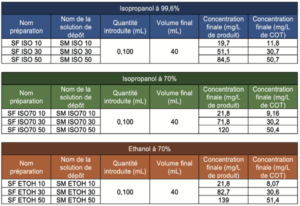
Step 1: Deposition of 100 μL of ethanol or isopropyl alcohol deposit solution prepared beforehand at a concentration that allowed the desired levels to be achieved, directly in a vial for TOC analyses.
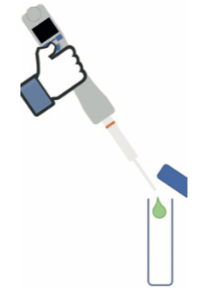
Analysis of samples in the TOC meter is sensitive to contamination (non-specific method), this is why evaporation of the 100μL samples was conducted naturally, without heating or airflow. The TOC vials were placed open under an extractor hood to assist evaporation without inputting external contamination.
Despite all these precautions, and in order to take account of potential carbon contamination appearing during sample evaporation, sample blanks were also prepared and left to evaporate beside the test samples.
Step 2: Deposition of 100 μL of ultrapure water used to prepare the alcoholic deposit solutions, used for the preparation of samples.
Step 3: The vials prepared in steps 1 and 2 were left until the deposits evaporated completely
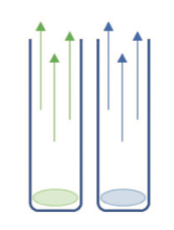
Step 4: After total evaporation, 40mL of water was added to the dry vials to obtain a volume equivalent to that of the reference samples and so allow the results obtained to be compared. The same quantity of the same ultrapure water was added to the evaporated blanks to determine the TOC content of the ultrapure water added to the evaporated samples, as well as the potential quantity of carbon contributed by the evaporation step. For each test series, 3 vials of evaporated blanks were prepared to obtain a more robust TOC content value. The mean of the 3 results was used to deduct TOC content from the evaporated samples.
The nature and quantity of each sample prepared, per series, are summarized below:
4. Results
From the results obtained for all of these preparations, percentage evaporation was calculated in accordance with the following equation:
4.1 Results obtained for the analysis of blanks
The mean of the carbon content of blanks for each type of blank and for each series is presented below:
As evaporation was not facilitated by any intervention, in order to avoid any contamination, it consequently required a significant period of time (around 24h).
This is why water could not be added to the evaporated samples and blanks on the same day as the reference solutions and blanks. So, differences between the values of blanks used for corrections are expected and directly linked to the quality of the water used on the day of solution preparation.
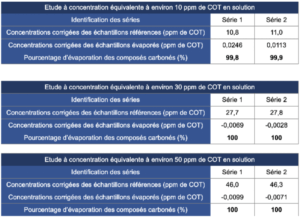
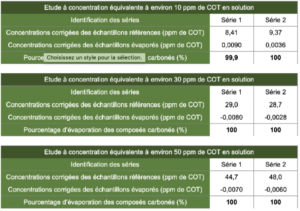
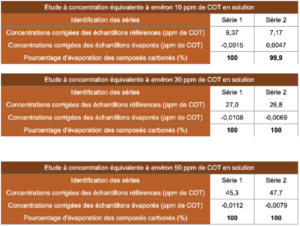
4.2 Results obtained for the study of 99.6% isopropyl alcohol
4.3 Results obtained for the study of 70% isopropyl alcohol
4.4 Results obtained for the study of 70% ethanol
Conclusion
The concentrations obtained for the reference solutions were close to the expected concentrations. Thus, the reference solutions are representative of deposits and do not display bias.
The concentrations of evaporated samples corrected by the concentrations of evaporated blanks provided results close to a nil value. This means that the TOC vials that contained a known quantity of alcoholic solution, once evaporated, provided carbon content values similar to those supplied by control TOC vials.
The evaporation efficiency calculated according to the formula presented in the introduction to the paragraph “Results” is located between 99.8% and 100%, that is almost total evaporation. These results thus allow the conclusion that the alcoholic solutions studied in this study do not generate carbon-containing residues after evaporation on glass. These conclusions cannot be transposed to another material without carrying out additional studies. It is also prudent to specify that the results obtained in this study are just an observation derived from the products studied and that product formulations can vary (different quantity and nature of additives). In this sense, it is important to check that each product used does not generate residues after evaporation and that their formulation is based on pure products known to be volatile such as isopropyl alcohol or ethanol.
Share article

Eva Bellanger – Cophaclean
Titulaire d’un master, Eva est spécialiste en analyses chimiques à l’état de traces depuis 9 ans. Elle est responsable d’activités analytique chez Cophaclean, société spécialisée en consulting en industries de Santé. Elle intervient pour la réalisation des projets analytiques, formations ou en tant que support technique.
eva.bellanger@cophaclean.fr
Acronyms
TOC: Total Organic Carbon
EtOH : Ethanol
SS: Stock Solution
DS: Dilute Solution