Summary
- Current state & trends for Single-Use technologies implementation in the Biopharmaceutical Industry
- Continuous Processing. Performance Enhancements for Perfusion Applications in 50L to 500L Single-Use Bioreactors: A Technical Comparison of Performance Characterization, Cell Culture & Scale-Up Modeling
- Implementation of Single-Use in Drug Substance filling before transportation: Product Development case study
- Single use connection technologies: current situation and trends
- Extractables and Leachables from SUS - aspects beyond Extractables Measurement & standardization.
- Toxicological evaluation of extractables and leachables associated with the use of Single Use Systems (SUS)
Faced with a boom in the use of single use systems, the transfer of liquids during different unitary operations has generated new needs to provide connections and interoperability between equipment. In fact, although connectors remain one of the oldest single use devices alongside bags and filter cartridges, it should be noted that significant efforts have been made by suppliers to perfect these components.
So, new materials are used to ensure better compatibility with the physicochemical constraints of processes, testing and documentation of these systems have been particularly strengthened to meet the expectations of users and health authorities.
In the first part, this article aims to depict a panorama of the technologies available today and the associated developments. The next part will endeavor to present the performance and quality criteria of connectors and practical aspects of their use. The last part will present the trends identified by the working group.
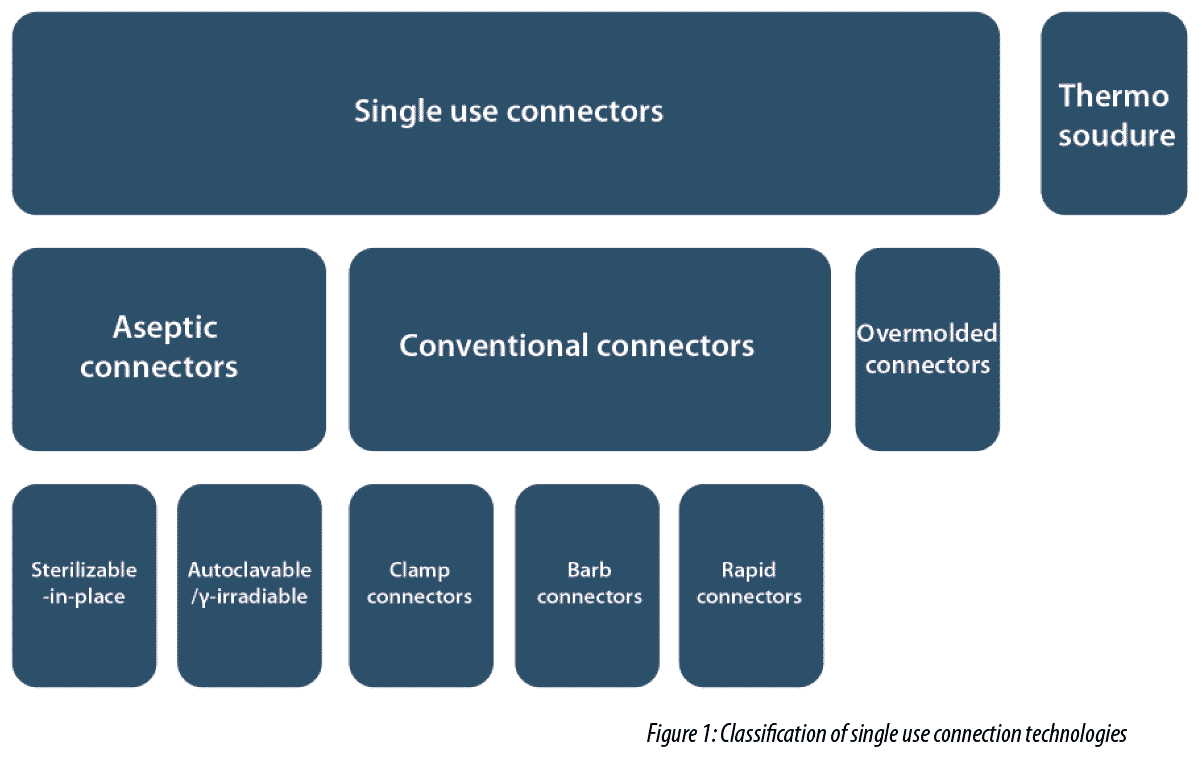
1. Presentation, classification and terminology of connection technologies
Connection technologies can be divided into different families according to the device implemented and the application for which it is intended. (Figure 1).
Conventional connectors are adapted versions of existing reusable technologies. In particular we find barb connectors and clamp connectors used for a long time as standard connectors in the pharmaceutical industry on stainless steel equipment. In this category, we also find rapid connectors which enable connections to be made and locked without accessories or additional tools.
Currently there are many proprietary variants of aseptic connectors. These safeguard the connection between two flexible tubes by preventing exposure of fluid to the atmosphere of the working environment. The most recent products allow several connection/disconnection cycles and their ergonomics have been perfected.
Finally, two connector technologies stand out in particular and consist firstly of connectors over-molded directly by the manufacturer onto tubing and secondly thermal welding technology, which allows direct tube to tube connection/disconnection of tubing in compatible polymers.
1.1 Conventional connectors (clamp, rapid connectors and barb connectors)
The single use connector models most used currently are the following connectors: barb connectors, Triclamp fittings (TC, triclover…) and rapid connectors (MPC/MPX…).These connectors are traditionally used as they are polymer adaptations of already existing stainless steel connectors considered standards in the industry. These are older, are less subject to intellectual property considerations, and are available from a relatively large panel of suppliers. In addition, they have developed successfully especially with respect to the materials used and tend to be more ergonomic. These connectors are also one of the solutions favored by bag suppliers, who often install these types of connectors by default according to the volume of the bags and the expected flows. In fact, for large diameter tubing, clamp and barb connectors are generally preferred although aseptic connectors are now available for large sections.
Barb connectors
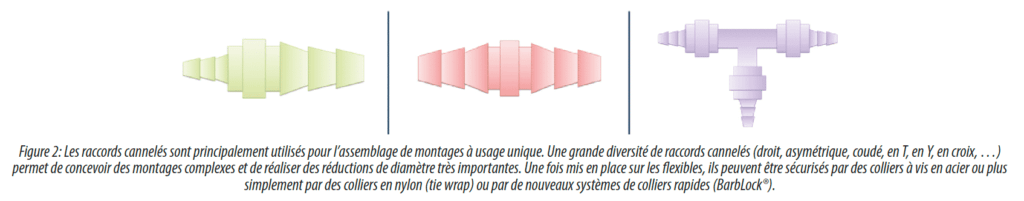
Barb connectors (and multi-diameter fir tree or nipple variants) are currently the standard connectors for assembly connections(Figure 2). In fact, a large number of formats are available and these allow for the design of complex assemblies that are sometimes tricky or costly to implement in stainless steel (large reductions in diameter, multi-way connectors, T connectors, Y connectors, elbow connectors with various angles…). These connections are permanent connections which are not intended to be disassembled. This connection type is therefore also used to connect most other families of connectors to tubing.
Innovations in these products focus notably on devices for securing tubing: collars, clamping rings, but also in the form of grooving and tips which allow us to dispense with collars or additional accessories. These connectors are now available in a wide variety of materials, ranging from polypropylene, medical nylon to fluoropolymers.
Clamp connectors
Clamp connectors are connectors traditionally used on the stainless steel systems used in pharmacy. In fact, they have been subject to significant standardization (GAMP/ISO) to facilitate interoperability between the systems used on production sites. Strengthened by significant experience in the design but also the expertise associated with their use by operators in workshops this type of connector has remained a standard which enables the setting up of hybrid systems (stainless steel/single use) in very large flow ranges. Nevertheless they remain complex and require a certain dexterity since it is a question of holding together the two clamp connectors, an intermediate gasket and the assembly clip at the moment of connection. The most recent developments focus on the clip (e.g. Biopure, Saint-Gobain) and on the direct overmolding of the gaskets in the connectors (TBL plastics) or on the tubing (Advantapure, Saint-Gobain, Parker…). Nevertheless, these systems involve significant open phases during connections/disconnection which limits their use on sterile single use apparatus. Use of these systems will probably decline in the face of aseptic connectors in applications where contamination must be controlled.
Barb connectors
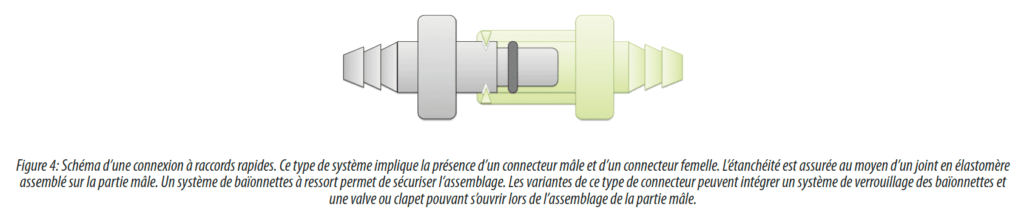
The development of rapid connectors was accelerated by the development of single use components in particular bags and bottles. In fact, resistant to the sterilization methods of these consumables (generally by gamma irradiation), they were quickly selected by most manufacturers for their practical aspects and their ease of use. They offer connection reversibility and excellent ergonomics for assembly or disassembly. Moreover, there are adapters to sterilize them in place or to allow direct use with clamp connectors. Some versions with valves are also often used for critical applications without however claiming to be aseptic since there is an open phase (although reduced).
The number of suppliers of these solutions has for a long time been reduced but new players are bringing alternative technical solutions that are compatible and interoperable with existing connectors.
Overmolded connectors
Overmolded connectors are connectors molded directly from the elastomer used for the tubing used in assemblies. These connectors present the great advantage of drastically reducing the risk of leaks and problems linked to the integrity of assemblies at the connections. Further, they permit the design of complex assemblies that the size of traditional connectors would not allow to be installed (e.g. multi-way caps, cross fittings and reducer fittings, manifold /series of connections…). However, the use of these assemblies often involves a customized design and complete outsourcing of the assembly construction. Thus these systems impose supply constraints that need to be anticipated since the number of suppliers remains relatively limited. Equally, physicochemical compatibility with the process must be carefully studied since the choice of materials is also reduced (silicon or thermoplastic elastomer). As these materials are flexible, their pressure resistance may prove limited for some applications. In these cases the use of overmolding on reinforced tubing (simple or double weave) should be planned. More generally, the implementation of such systems must be anticipated from the point of view of qualification/validation and be the subject of a close collaboration with the supplier…
1.2 Aseptic connectors
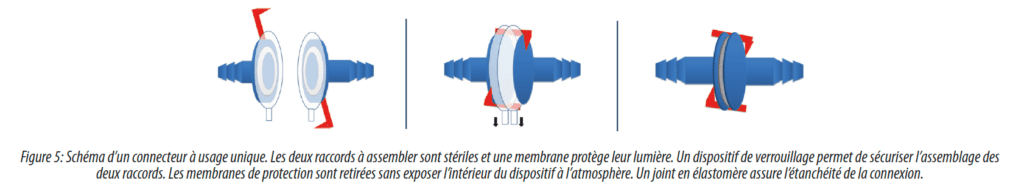
Aseptic connectors are the most numerous type of connector. Their use has grown significantly during the last ten years because of the possibility they offer of maintaining the sterility of connections, combined with an ease of use comparable to that of rapid connectors. More recent, these connectors have for the most part benefited from the development of materials, making them compatible with all solution preparation, biological culture and purification operations, as well as sterile product filling operations.
The main manufacturers of single use systems have developed aseptic connectors (Merck Millipore, Sartorius, Pall Biotech) it is interesting to note the existence of an independent manufacturer (Colder). Most of these manufacturers now develop or market their second generation connectors which bring some innovations: ergonomics, possibility of connecting/disconnecting connectors several times, finally the possibility of sterilizing single use connectors in place so allowing the combination of single use systems with sterilizable-in-place installations (e.g. single use sampling assembly on a stainless steel tank).
This relatively large solutions offer however conceals great disparities especially in design: the user must be vigilant with respect to the compatibility of the materials with their application, the design male/female or universal, the possibilities for diameter reduction… Other parameters are also to be taken into account such as the documentation produced by the manufacturer and the tests they have carried out, product availability and supply channels.
1.3 Connection by thermal welding
This relatively old technology is based on thermoplastic elastomer tubing (C-Flex®, Advantaflex®, Pharmed®…). The ends of the two tubes to be welded are inserted into the dies of a welding machine which will hold the tubes and weld them by heating the polymer to temperatures in the order of 125-200°C. Welding time varies with the wall thickness and the diameter of the tubes.
The principle allows the exposure of an open phase to be limited and today some systems are used for aseptic connections. The advantage of these systems is to be able to connect and disconnect the tubing virtually an infinite number of times.
Thermal welding nevertheless involves certain specific constraints and risks that are to be controlled such as the impossibility of making reductions in diameter, generation of particles in the tubing and the correct training of operators.
1.4 Other connectors
Other connectors are commonly used for some operations. So, Luer connectors are still much used for sampling or the introduction of small volumes in processes. They have benefited from innovations such as for example valve systems which limit the open phases.
Also, for a proportion of utility connections, compression connectors (push-in, e.g. Legris CleanFit) are very often used and are now available in materials compatible with the requirements of the Pharmacopeias.
2. Performance, quality, and practical aspects of connection technologies
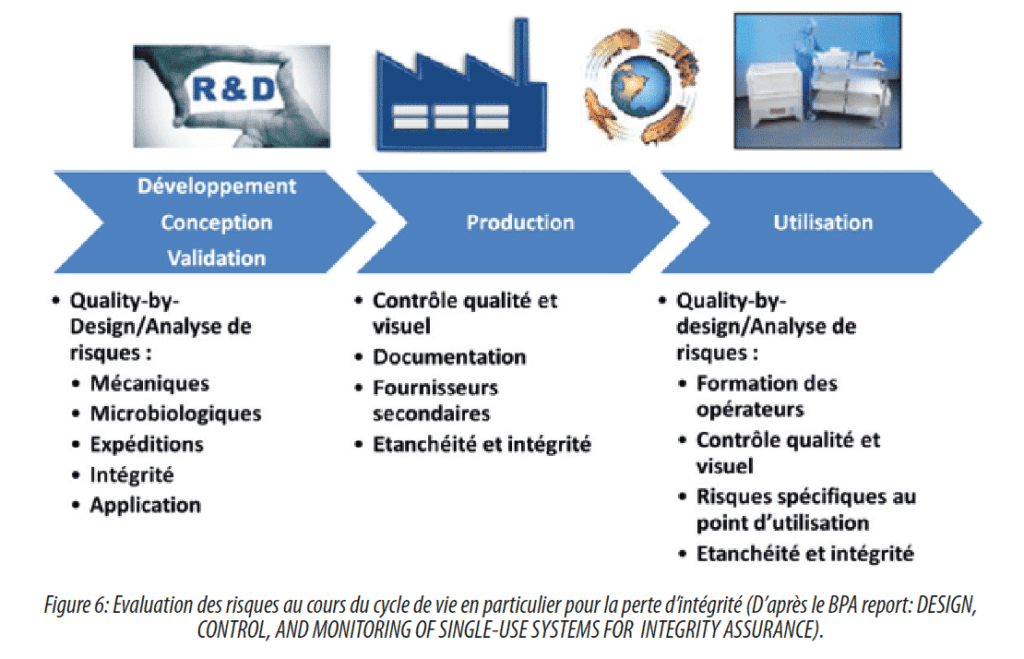
In order to cover the practical aspects of connection technologies, it is important to start with an analysis of risks encountered through the use of these connections in the manufacturing process(see Figure 6).
Some points to be taken into account are general and applicable to all manufacturing processes, and particularly the following cases:
- Control of connector manufacture: critical dimensions, control of critical production parameters;
- Control of the transport of components to the final user:
- Functional aspects: flow range, resistance to pressure, temperature of use, number of connections and time acceptable to make a connection;
- Chemical compatibility of these connections with the products present in the manufacturing process;
- Ease of connection(for example: number of steps, less flexible male/female connection…);
- The risk of the presence of particles (influenced by the working environment)
- E&L-Compliance with pharmacopoeias.
Other points are more specific and dependent on manufacturing processes:
- Presence of a cleaning step – resistance to cleaning agents.
- Presence of a sterilization step – resistance of connectors to sterilization processes (for example: autoclaving or gamma irradiation).
- Sterile process – data associated with asepsis (for example leak test, security of the connection…)
The risks identified may be covered by controls at manufacturer level and/or user level.
The general risks are linked to problems of compatibility, the presence of extractables or risks of potential contamination (particles, endotoxins). Information documenting these risks is generally available from the suppliers.
For the more specific risks, supplier data represents fundamental information which must be studied by the user. This initial study will support the introduction and qualification of the connector in the final manufacturing process. Typically, for risks associated with sterilization and use in sterile manufacturing, it is recommended to refer to supplier data and site practices to evaluate the capacity of the connector to tolerate the cleaning and sterilization process and data supporting use of the connector in an aseptic environment (autoclaving or gamma irradiation sterilization data, biological compatibility data of the connector and the connection, leak test in routine testing).
On this basis, the final user will carry out their qualifying operations and routine testing (for example leak tests of connections during manufacture ).
3. Trends observed in connection technologies
3.1 New materials
The materials of single use connectors are plastic polymers shaped either by injection or machining. Depending on interactions with the product and process constraints, it is necessary to be vigilant regarding the materials of the connectors selected.
Easy to use, polypropylene and polycarbonate are polymers used both for product contact and connector structure but are relatively sensitive to solvents. Their use is therefore less indicated in the preparation steps of aggressive solutions, but they remain compatible with biological culture conditions. The sterilization conditions should be carefully studied as temperatures above 125°C may damage their physical integrity (creep, deformation) and weaken them.
Chemically, fluoropolymers are much more resistant and tolerate sterilization temperatures in moist heat perfectly. PTFE is used for small parts as it is relatively flexible but it is sensitive to radiation (sterilization with gamma rays). The more expensive PVDF is mechanically very resistant and better tolerates ionizing radiation.
Also expensive, polysulfones are replacing polycarbonates with very good mechanical properties and excellent chemical compatibility. These polymers are also compatible with chlorinated substances. Given the price of the material, polysulfone connectors are often more expensive than their equivalents in polypropylene or polycarbonate. For large diameter connectors, this polymer is sometimes strengthened with glass fibers.
Silicon and other thermoplastics are used for overmolded parts and for the leak-tight parts of connectors.
All the materials cited above are generally formed by molding and plastic injection. The use of other polymers such as Polyetherimide (PEI) and Polyether Ether Ketone (PEEK) is more recent. These extremely resistant materials must be machined. They are used particularly in sterilizable-in-place connectors or with the use of steam, they are exposed to very strong temperature and pressure constraints.
The use of physically more resistant materials allows new applications such as sterilization-in-place as well as chromatography steps or other unitary operations under pressure. Moreover, these new materials, more chemically inert, generate fewer extractables and leachables.
3.2 The number of connections
This point is an area of development for aseptic connectors in particular. In fact, the first aseptic connectors had only single connection functionality which could not be repeated. Intermediate products allowed for a complete connection/disconnection cycle. Now, the most recent products claim repeated connection/disconnection/reconnection cycles. This trend is particularly true with the renewal of interest in thermal welding which offers large financial benefits by significantly reducing the cost per connection. This trend leads to another development dealing with connection integrity.
3.3 The integrity of single use connections
The testing of the integrity of single use devices is an important subject and connection technologies do not circumvent this requirement. As the value of products is constantly changing, the leak detection, and their anticipation in advance, are the subject of development and innovation by manufacturers. In fact, beyond manufacturer factory tests where sometimes 100% of parts are checked, integrity is considered from development onwards: the ergonomics of connectors, elements providing proof of connection (click or visual). But it is also with the use of new integrity test systems where assemblies are tested as a whole that the connectors are evaluated. As leak tests are incompatible with aseptic connectors before they are connected, these connectors can be tested for pressure resistance once installed in a single use assembly. This point is particularly important during the implementation of sterilizing filtration assemblies with an in-line test of the integrity of the sterilizing filter.
3.4 Connector controls and documentation
Although single use bags have received most attention regarding the issue of container/content interactions, the lessons and practices drawn from work on these products are now being applied to connectors. So, risk analyses should be implemented with the objective of rationalizing the study of components depending on their criticality in the process. For connectors, contact time is often reduced to transfers but the presence of critical extractables/leachables identified in other single use systems during the process implies particular attention, and analytical work similar to that performed on bags will be expected. Further, when connectors claim new functionalities, in particular the sterility of the connection (even several connection/disconnection cycles), they are exposed to a need for additional documentation and validation.
The part dealing with materials and extractables/leachables is expected by the customer to be included in the tests performed by the supplier. The study methodologies proposed by various working groups such as the BPOG(1)or the BPSA(2), tend to be more routinely used by suppliers but they do not necessarily deal with all existing systems. Likewise, extensive work on the new functionalities of connectors should be performed by the user. Not to mention that the use of new connectors and their documentation can highlight practices or lead to as yet unresolved questions on other connection methods implemented (operator training, operation of aseptic connections…). The implementation of single use connection technologies should not therefore be neglected and real work on documentation and validation will be expected both by the authorities and the internal quality and regulatory teams.
4. Single use connections: developments and revolutions
As has been seen in this article, connection systems are multiplying and developing. On one hand, systems with a sometimes old design are benefiting from real technical innovations which are reviving their value in some operations associated with single use systems. On the other hand, the revolution in aseptic connectors is continuing, bringing with it new practices that reinforce manufacturer controls and impose on users the requirement to better study the incorporation of devices that are a priori minor.
Risk analyses are at the center of single use implementation and connectors do not escape this. This panorama produced by A3P within a Single Use common interest group (GIC) led its members to reflect on the connector selection process of users. Following its research work into the latest developments, the group is now working to develop a methodology for selecting connectors according to their application.
Share article

Sylvain PEYRACHE
sylvain.peyrache@gmail.com

Charlotte MASY – GSK
charlotte.l.masy@gsk.com
Definitions
(1) BPOG : the BioPhorum Operations Group is a working group that brings together industrialists from the pharmacy sector and equipment suppliers.
(2) BPSA : the Bio-Process Systems Alliance is an alliance that promotes the incorporation of single use systems