Sommaire
- Les systèmes de confinement : Sources de sécurité et d’un business amélioré
- Stratégies de mise en place et de gestion de la bioproduction selon les BPF
- Disinfectant Validation : a roadmap for regulatory compliance
- Cahier Pratique : Des outils de résolution de problèmes dans le cadre d’un programme d’amélioration continue de la Qualité
- Collaboration entre Transgene & Pall Life Sciences pour le développement d’un procédé
- De l’importance des intégrations dans LIMS
- Freeze drying with collapse is not necessarily bad for stability and can reduce cost
- Prenez-vous trop de risques ? Adoptez la nouvelle approche qualité « QbD » pour vos cycles de lyophilisation
- Management – «Quelques pensées sur la direction de sites et le leadership, Partage d’expérience et de petits trucs…»
Lyophilization is often used to increase the stability and shelf life of proteins which are physically and/or chemically unstable in aqueous solution. Freeze drying removes water protein solutions, including water from the protein surface which plays a major role within the protein structure, and this frequently causes damage. An appropriate choice of stabilising additives is necessary to protect the protein from the denaturing during both the freezing and the dehydration. The components of the excipients displace water by replacement, which makes the drying possible without causing damages to the proteins. In addition the solidification of the excipients forms a matrix around the particles which keeps the protein in their native structures.
Concerning the process, the cycle is usually running below collapse temperature to avoid a loss of physical structure, an incomplete drying (high moisture content), a decrease of solubility (or increase of aggregation), or a decrease of activity and/or stability. In addition, the best freeze drying cycles are those that have been optimised to be as short as possible: run at high temperature to run fast. In that case, formulation should have a high glass transition temperature to facilitate freeze drying. Some disaccharides like sucrose are often good stabilisers for proteins but unfortunately they have a low collapse temperature.
Recent studies seem to indicate that drying above the collapse temperature is not necessarily bad; the protein stability is not always damaged. Our objective was to study the activity and stability of a freeze dried protein in vials which shows signs of collapse. The protein selected was the alkaline phosphatase (from Sigma Aldrich) which is used to help detecting liver disease. The enzyme may catalyse the hydrolysis of various monophosphate esters at alkaline pH, the method for the measurement of alkaline phosphatase specific activity was based on the conversion of para-nitrophenylphosphate (p-NPP) to para-nitrophenol and the colorimetric determination of the resulting coloured product.
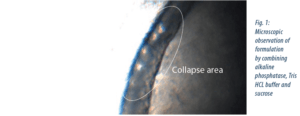
A formulation by combining alkaline phosphatase, Tris HCL buffer and sucrose was defined. The collapse temperature (Tcoll) was measured using freeze drying microscopy (cf. figure 1)
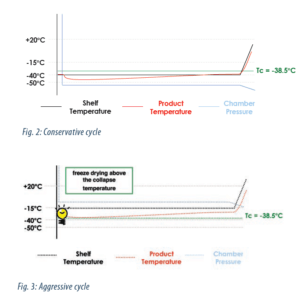
The formulation was poured into a vial and loaded in a freeze drier. Two cycles were running: one with a “conservative” freeze drying protocol which maintains the product temperature below collapse temperature (time of cycle : 72 hours, cf. figure 2) and an “aggressive” freeze drying protocol carried out at higher temperature and higher pressure which does not maintain the product temperature below collapse temperature (time of cycle : 48 hours, cf. figure 3).
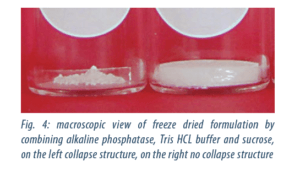
In the first case the appearance of the freeze dried products were good, in the second case they showed signs of collapse but the cycle was shorter (cf. figure 4).
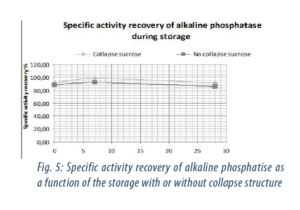
These measurements are performed in accordance with a TO and after storage at a 25°C temperature conditions. The results of storage stability in dried state (cf. figure 5) confirm that sucrose stabilizes the protein conformation against denaturing but more particularly the results suggest that the freeze dried product with signs of collapse did not damage the alkaline phosphatase quality. The specific activity recovery of alkaline phosphatase is quite the same with a collapse macroscopic structure than with none.
This presentation is for informational purposes only and it is not about new guidelines for stabilization of proteins by freeze drying but it suggests that in some cases, not generally speaking, we can freeze dry above the collapse temperature to have a short cycle which implies lower costs without causing damages to the product if we do not care to have a not elegant product, if the product aspect is not that important.