Sommaire
- La nouvelle réglementation européenne 2017/745 sur les dispositifs médicaux, les principaux points à retenir
- Optimisez et pérennisez vos investissements industriels
- Quels sont les enjeux d'une défauthèque de mirage ?
- Automatisation : l’analyse fonctionnelle, clé de la réussite d’un projet !
- Three steps to contamination control when utilising single use equipment
- Comptage de particules automatisé dans les tunnels à air chaud
- Hydrogen Peroxide Sterilization and Isolator Connections through a Magnetic Driven Door For Innovative Lay-outs in Aseptic Processes
- Fast X-Ray Tomography Techniques : ready for new Pharmaceutical Applications ?
Hydrogen Peroxide Sterilization and Isolator Connections through a Magnetic Driven Door For Innovative Lay-outs in Aseptic Processes
Nowadays, the barrier technology with isolators is the popular solution in aseptic processing. In particular, the increase in use of isolators is driven by the need to reduce contamination risk when handling items which are to be processed in controlled environment.

At the same time, the diffusion of vaporized hydrogen peroxide, as a powerful decontaminant, has further promoted the use of isolators in designing a sterile line. De Lama, based on their long and consolidated experience in sterilization equipment adaptation to the different needs of aseptic processes, has conceived innovative plant configuration solutions by coupling various sterilizers with isolators, taking advantage of a magnetic-driven door.
1.The use of isolators in aseptic processes
The expansion of the use of isolators is mainly due to their capability of containment and to their good flexibility in meeting the controlled environment requirements, which are present in aseptic process line.(1)(2)
The use of isolators is encouraged by regulatory bodies: the revised version of GMP Annex 1 (still under discussion) reports an expanded paragraph about isolators (and RABS), highlighting the critical factors associated with this technology and providing the basic requirements to be met for their application in sterile processes.
Among others, specific requirement is reported about the determination of risk factors in developing a decontamination, a disinfection or a sterilization process, associated with the manufacturing operations and materials which would be present within the isolator.
The use of hydrogen peroxide in its vapour-phase status (VPHP) as a disinfectant and decontaminant in isolator has become common practice. In fact, during the last 10-15 years, vapour-phase hydrogen peroxide (VPHP) generators have been developed and integrated with isolators and material pass- boxes, as a consequence of the expansion of barrier technology.
Large production isolators now utilize integrated generators that work in concert with their air-handling systems to distribute and purge hydrogen peroxide, resulting in short, effective decontamination cycles.
By adopting this approach, hydrogen peroxide, in its vapour phase, would be recognized as a sterilizing agent, which could not only decontaminate the internal enclosure of the isolator but also sterilize the materials which would be enclosed.
Notwithstanding these evident advantages, regulatory authorities have not supported the use of VPHP sterilization process yet. Their concerns are mainly founded on the “fragility” of the process itself, taking into account the difficulty to achieve VPHP penetration in all parts of items to be sterilized and still the presence of high degree of risk in managing a VPHP sterilization procedure for critical items.
In the last few years, the limitations in using VPHP as a sterilizing agent have been overcome with the development of sterilizers where suitable operating conditions are applied for a batch sterilization process, at low temperature.
The basic VPHP sterilization cycle consists of three fundamental stages: vacuum generation, H2O2 injection and aeration. The whole process takes approximately 2 hours, including aeration time (removal of H2O2). After the aeration is complete, the sterilized items are removed.
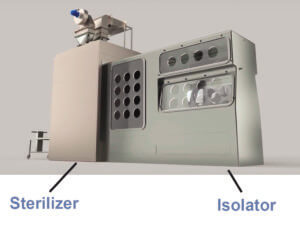
2. Isolator and sterilizer connection: innovative plant configurations
Taking into account the different needs of aseptic processing flows, De Lama has developed innovative aseptic line configurations by coupling isolators with various sterilizers.
These innovative configurations include the use of a magnetic-driven door (MAGNETODOOR®)(3) that is able to connect sterilizer and isolator through a system that is easily cleanable and ensures a reduced risk of contamination.

These different configurations allow to design a sterilization process which can be applied to either heat-resistant or heat-sensitive products and materials, before their introduction in an isolator, depending on the use of a standard steam sterilization, a dry heat sterilization or a low temperature VPHP sterilization process.
The three main innovative layouts, based on sterilizer/isolator connections through magnetic-driven doors, are the described here below, highlighting their advantages in the different aseptic processing requirements. Additional plant configurations can also be conceived, by combining more than one of these layouts.
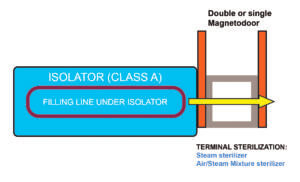
Configuration (A)
Connection of the sterilizer to the loading side of the isolator
This configuration includes a double magnetic-driven door, allowing to load the items into the sterilizer and to unload the sterilized items directly into the isolator, by the presence of a tight connection between the two pieces of equipment.
The peculiarity of this configuration is its flexibility, as it can easily be adapted to specific process needs. In fact, the sterilizer can be one of the following options: steam sterilizer, air/steam sterilizer, dry heat sterilizer, VPHP sterilizer or a piece of equipment that combines washer/steam sterilizer or washer/VPHP sterilizer.
According the choice of the sterilizer, this configuration can be applied, for instance, to the following operations, which are requested for aseptic processing of various items:
• washing and steam sterilization of heat-resistant parts of the aseptic line, which are to be used in the isolator, where the filling process is carried out,
• de-pyrogenation of containers, which are to be used within the isolator,
• VPHP sterilization of heat-sensitive parts of the aseptic line, which are to be used for the filling process in the isolator.
It is, in particular, to be observed that the direct connection of a VPHP sterilizer with the isolator allows to apply a sterilization process to all items which can’t be reliably decontaminated and sterilized within the isolator, due to the uncertain penetration of VPHP when used at atmospheric pressure.
Depending on the process flow, a pass-box can also be connected to the VPHP sterilizer through the double magnetic-driven door, to decontaminate any heat-sensitive items before loading it inside the isolator.
The evident advantages of this configuration are risk contamination reduction and improved process flow. In fact, the direct connection of the sterilizer (any type) to the isolator reduces the risk of contamination of all ancillary items after sterilization, which are to be loaded inside the isolator. At the same time, this solution would reduce the lead time for the preparation and transportation of ancillary items from sterilization point to inside the isolator.
The additional advantage is linked to the use of the magnetic-driven doors. If compared to the traditional doors, the following features are to be highlighted: space reduction, compatibility with hydrogen peroxide, reduction of mechanical replacement parts, improved cleaning due to the absence of hidden points, difficult to be reached.
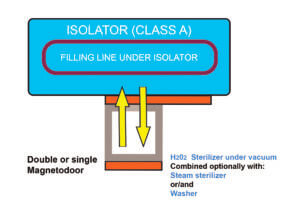
Configuration (B)
Connection of the sterilizer to the unloading side of the isolator
This configuration includes a double magnetic-driven door connecting the unloading side of the isolator with the sterilizer. This would allow to directly unload items from the isolator into a sterilizer, by the presence of a tight connection between the two pieces of equipment.
Even in this case, a flexibility of application of this configuration is maintained, taking into account that the sterilizer can be a steam sterilizer, an air/steam sterilizer or a VPHP sterilizer.
As an example, when the isolator is connected to a VPHP sterilizer, this solution allows the terminal sterilization of sealed packages containing products which are heat-resistant or heat-sensitive, after their aseptic processing inside the isolator.
The same advantages as described for Configuration (A) can be reported: reduced risk of contamination, saving of space, optimization of process flow.
Configuration (C)
Connection of the sterilizer to the loading/unloading back-side of the isolator
This configuration includes a single or a double magnetic-driven door, connecting the sterilizer with an alternative loading/unloading side of the isolator.
This configuration has been conceived considering any need to move items from the isolator into a sterilizer and back into the isolator when the sterilization process is finished.
According to the aseptic process needs, the sterilizer can be, as in the previous configurations, a steam sterilizer, an air/steam sterilizer, a dry heat sterilizer, a VPHP sterilizer or a piece of equipment that combines washer/steam sterilizer or washer/VPHP sterilizer.
The particular application of this configuration is for moving ancillary items, which are used in processing (e.g. in a filling line installed inside the isolator) directly from the isolator chamber into a sterilizer, without the need of taking them out from the isolator in the clean-room. This direct connection allows to get back the items into the same isolator as soon as they have been sterilized.
The main advantage of this configuration stays in the reduced request of space in the clean-room, an optimization of process operations, always keeping low the risk of contamination, by using the direct connection between the sterilizer and the isolator through a magnetic- driven door.
3. Conclusive remarks
The use of isolators in aseptic process operations reduces the contamination risk and also increase the worker safety when highly potent drug products are handled. An expansion in using isolators is expected also due to the positive attitude of regulatory bodies on this technology.
Isolators show a good flexibility and can be quite easily installed in an aseptic facility, where they can be used for product transfer or for specific operations, mainly product aseptic filling and finishing.
However, the installation of isolators in a facility would require to consider their connection with the sterilization units which are used. In fact, taking into account the limitations in using isolators for sterilization through VPHP, there is very often the need to move items from isolators to sterilizers and back to isolators for ensuring the sterility compliance in an aseptic processing flow.
The direct connection between an isolator and a sterilizer represents a significant advantage as it allows to reduce the time for transfer operation, keeping the contamination risk at low level.
According to the aseptic process being operated, different connections between isolators and sterilizers can be designed and installed. In the above described layouts, the key element is the magnetic-driven door, which has been designed to be compatible with hydrogen peroxide (in case of a connection with a VPHP sterilizer), to have reduced mechanical replacement parts and absence of hidden points, ensuring full decontamination requirements.
These innovative plant configuration solutions are expected to be more and more adopted in designing an aseptic process facility, taking into account the evident positive impact in terms of better contamination control, reduced space required in the operating area and reduced operation time, with a consequent cost containment.
Partager l’article

Dr. Piero LAMARTINO – PHARMACEUTICAL CONSULTANT
After working for 40 years in the pharmaceutical industry, gaining experience in dosage form development and production, and taking responsibility of quality and plant management (including API manufacture), he acts now as an independent consultant.
References
(1) J. Markarian, Aseptic Processing Innovations on Display, Apr 17, 2019
http://www.pharmtech.com/aseptic-processing-innovations-display
(2) F. DeSantis, L.Folks, Aseptic Formulation and Filling Using Isolator Technology, Pharmaceutical Technology 2003
http://files.pharmtech.com/alfresco_images/pharma/2014/08/22/24a81ec5-2a6a-4c9f-9895-284c0fc72f69/article-67145.pdf
(3) Patent Pending