Sommario
- Injectable Combination Products. Issues and challenges for industry.
- Deadline Extension or Not: Key Points for Class I Manufacturers
- The new challenges of injectable medicines
- Clinical Investigation of Combined Medical Devices Under New Regulation (EU) 2017/745 (MDR)
- Contribution of the physicochemical characterization of the materials constituting medical devices for rationalisation of their biological assessment.
- Steering Cleaning Validation Performance : A Key Industrial Challenge.
- Cleaning Process Validation: Why and How to Validate Analytical Methods and Related Sampling Methods
- A Risk-Based Approach to Stainless Steel Equipment Maintenance in cGMP Manufacturing Environment
- Single Use Systems vs Re-Usable Stainless-Steel Equipment. Compliance & Quality Perspective.
Injectable Combination Products. Issues and challenges for industry.

Historically, development of injectable combination products has been driven by therapeutic areas such as diabetes, growth hormones, anticoagulants or vaccines, in which injector pen or pre-filled syringe solutions have demonstrated their efficacy, as well as a good level of adherence among patients and health professionals.
In the last few years, however, the pharmaceutical industry has focused its R&D efforts on new biological entities (e.g. therapeutic proteins, monoclonal antibodies) to treat cancer or chronic inflammatory, autoimmune, cardiovascular or neurological pathologies. These products are no longer being marketed for intravenous administration in hospitals by a health professional, but for subcutaneous administration by the patients themselves in their homes.
The challenge for industry is to come up with patient-centric solutions that enhance the efficacy, safety and tolerance of the treatments, for a controlled overall treatment cost.
1. A complex and ever-changing regulatory framework
In the United States, the term “Combination Products” is defined in 21 CFR 3.2 and comprises three classical cases: a product composed of at least two components mixed and marketed in a single entity, two or more products packaged together in a kit, and products that are packaged separately but the labels of which specify their use together to achieve the effect being sought (cross-labeled products). The FDA department dedicated to combination products, the OCP, refers the products to the relevant evaluation service (CDER/CBER for drugs, CDRH for medical devices) according to the product’s primary mode of action (PMOA).
As regards the quality system requirements for an injectable combination product, the following main texts apply: 21 CFR Part 210 & 211 (for the drug part) and 21 CFR Part 820 (for the medical device part).
Two approaches are therefore possible: either the elaboration of a system in full compliance with the standards applicable for each component of the combination product, or a rationalized approach based on full compliance with “Drug” cGMP and including the specific elements of the medical device quality system (control of design and development, control of purchasing, management responsibilities, CAPA, associated services, installation activities).
At European level, the status of combination product is not defined. It is either a Medical Device or a Medicinal Product. The factors taken into account to rule on the product are to be found in Article 1 of the new European Regulation 2017/745 on medical devices. If the device contains a medicinal product whose action is ancillary to that of the medical device, then the product is regulated as an MD. If, however, the device is intended specifically to support the medicinal product, then the product is governed by Directive 2001/83/EC on medicinal products.
In June 2019, the EMA published a draft guideline on drug-device combinations for consultation, with a view to clarifying and harmonizing the regulatory requirements for these products in modules 1 and 3 of MA dossiers. The definition of drug-device combinations (DDCs) is similar to the FDA approach in that a distinction is made between “integral” DDCs (meaning a “single entity”) and “non-integral” DDCs (co-packaged and cross-labelled).
For a “non-integral product”, such as a rechargeable injector pen or a subcutaneous infusion pump, the MD part must bear the CE marking according to Regulation 2017/745 applying the requirements of classes IIa or IIb (Article VIII – rule 12) with the involvement of a notified body in the certification process. Manufacturers must therefore take account of the impact of this new regulation and the stricter requirements, notably concerning clinical evaluation, enhanced traceability by the introduction of the unique device identification (UDI) and post-market surveillance.
For a pre-filled administration system, the drug-device combination will be regulated as a medicinal product subject to Directive 2001/83/EC, while the injection device will still have to comply with the essential safety and performance requirements set out in Annex 1 to Regulation 2017/745 and include them in the MA dossier pursuant to Article 117 of this regulation.
2. Organizational, cultural and human challenges
Against this backdrop of major changes to the market and regulatory environment, industrialists find themselves facing new organizational, cultural and human challenges revolving around the management of the life cycle of their products.
The differences in standards, management systems, skills, technical language and terminology all constitute challenges to be taken up if time to market and the quality of the products that are marketed are to be controlled.
An integrated organizational approach must be implemented combining the requirements applicable to the lifecycle of a medicinal product and to that of its administration device, backed up by a robust quality system and methodologies allowing proactive management of the risks inherent to the marketing of highly complex products responding to significant needs of high-risk patients.
Drug-device combination products must therefore be developed in line with the requirements of medicinal product regulations, but must also include the design control, risk management and fitness for use of medical devices.
This integrated approach implies that the specifics of the administration device and its components be taken into consideration sufficiently early on and in an iterative manner in the development process of the combination product which is a complex system in which the interactions between the different components and sub-systems are critical to clinical performance and patient safety.
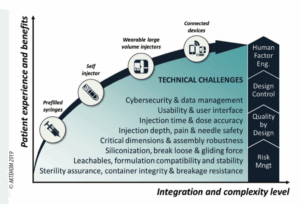
3. Technical and methodological challenges for an optimal patient experience
The needs of patients in terms of treatment safety and efficacy and also of user experience are leading industrialists to develop increasingly integrated and complex solutions, with each generation of drug-device combination products bringing new benefits, but also technical challenges and the risks that go with them.
Right from the very first stages of development, industrialists find themselves facing issues of the interaction between the formulation, primary packaging and administration device, and quite aside from the question of technical expertise, the effective implementation of the processes of risk management, quality by design and design control is essential to ensure control over the quality and safety of use of combination products.
Particular vigilance must be focused on the risks of compatibility between the formulation and the primary packaging items, notably regarding the potential toxicity of leachables from the raw materials or issues of the physicochemical stability of the formulation (aggregation, adsorption, degradation, etc.).
Functionality must be ensured by control over the critical dimensions of all the components, particularly at the interfaces between the primary packaging of the medicinal product and the administration device. For parenteral administration, control is required over the robustness of siliconization, filling and mechanical or even electromechanical assembly processes, in order to guarantee all the functionalities of the combination product.
The choice of components, specifications and product/process control strategy must be made based on an assessment in particular of the risks associated with poor control over injection force and time, and over the precision of the dose that is delivered, particularly for very viscous formulations.
Another major aspect of the marketing of combination products is fitness for use and the human factors, which are essential not only to ensure that the product is used safely, but also to create a preference for use of such products among patients and foster adherence to the treatment and hence its efficacy.
Via studies of human factors, the way users will interact with the product can be anticipated, understood and optimized in order to avoid use errors and limit the risks linked with inappropriate handling.
Analysis of use error risks in the very first design stages followed by conducting studies provides supporting data on the safety of use of the product and the benefits it brings, so that the design choices that have been made can ultimately be validated.
Conclusions & perspectives
Therapeutic and technological innovations are major drivers of the development of injectable combination products.
The complexity of these products and reinforcement of the regulatory framework require significant organizational and cultural changes of industrialists, as well as an integrated approach to the lifecycle of these products and cutting-edge multi-disciplinary expertise, backed up by robust development and risk management methodologies.
The texts and guidelines on drug-device combination products are relatively recent and changing, thereby opening up possibilities for global harmonization in coming years. Industrialists are also awaiting greater clarity and precision on the data that is expected in the regulatory dossiers, as well as on its presentation.
The development of new technologies and connectivity of the devices also brings new challenges revolving around data management and raises questions as to the protection of patient information, control over data integrity and system cybersecurity.
Industrialists will need to take account of these aspects and of the related regulatory changes in their risk management processes before they allow patients and healthcare stakeholders to benefit from these major innovations.
Share Article

Guillaume BONNEFOND – Aktehom
guillaume.bonnefond@aktehom.com

Loïc MENNRATH – Aktehom
loic.mennrath@aktehom.com
Glossary
CAPA: Corrective and Preventive Action
CBER: Center for Biologics Evaluation and Research
CDER: Center of Drug Evaluation and Research
CDRH: Center for Devices and Radiological Health
cGMP: current Good Manufacturing Practice
EMA: European Medicines Agency
MD: Medical Device
OCP: Office of Combination Products
PMOA: Primary Mode of Action
UDI: Unique Device Identifier
References
1. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use
2. Regulation 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices.
3. Quality requirements for drug-device combinations, (draft for consultation). European Medicines Agency; 2019.
4. Code of Federal Regulations Title 21, Parts 3, 4, 210, 211 & 820. Food and drug administration (FDA); 2019.
5. ICH Q8 (R2) Pharmaceutical development. ICH; 2014.
6. ICH Q9 Quality risk management. ICH; 2005.
7. ICH Q10 pharmaceutical quality system. ICH; 2008.
8. ICH Q11 Development and manufacture of drug substances. ICH; 2011.
9. ICH Q12 Technical and regulatory considerations for pharmaceutical product lifecycle management. ICH; 2017.
10. NF EN ISO 13485: 2016 – Medical devices – Quality management systems – Requirements for regulatory purposes. ISO; 2016.
11. NF EN ISO 14971: Medical devices – Application of risk management to medical devices. ISO; 2019.
12. ISO 10993-1: 2018 – Biological evaluation of medical devices Part 1: Evaluation and testing within a risk management process. ISO; 2018.
13. Technical Considerations for Pen, Jet, and Related Injectors Intended for Use with Drugs and Biological Products. Food and Drug Administration (FDA); 2013.
14. ISO 11608 (Parts 1-7) Needle-based injection systems for medical use – Requirements and test methods. ISO.
15. ISO 11040-8: 2016 – Prefilled syringes — Part 8: Requirements and test methods for finished prefilled syringes. ISO.
16. ISO 7886 (Parts 1-4): Sterile hypodermic syringes for single use. ISO.
17. NF EN ISO 62366-1: 2015: Application of usability engineering to medical devices. ISO.
18. Applying Human Factors and Usability Engineering to Medical Devices. Food and Drug Administration (FDA); 2016.