Sommaire
- Frontière Part I/ Part II des BPF : Modalités d’application aux produits biologiques
- Moving One Unit Operation At a Time Toward Continuous Biomanufacturing
- « Close Collaboration Maximizes Value of Engineered Solutions and Saves Time in Start-Up »
- Improving Single Use Bioreactor Design and Process Development. New Research Towards Intensifying Seed- Train & Scale-up Methods Using 5:1 Turn- Down
- Qualification approach for the validation of real-word shipping in single-use systems
- Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in BioBLU 0.3c Single-Use Bioreactors
- Single Use & Stainless Steel: complementarity or fight?
- Low Endotoxin Recovery (LER) is today one of authorities serious concerns regarding pyrogen testing
Low Endotoxin Recovery (LER), initially described by Chen and Vinther in 2013, is a phenomenon occurring in protein formulations that is characterized by efficient masking of Lipopolysaccharides (LPS/endotoxin). Depending on the protein – buffer characteristics, the temperature – and time – dependent masking effect can lead to complete non-detectability of LPS, which might present a serious risk for the patient.

At first being interpreted as a purely artefactual event occurring in testing laboratories, LER is today one of FDAs serious concerns regarding pyrogen testing.
Pyrogens – Definition and methods for their detection
Pyrogens: “A substance that induces a febrile reaction in a patient.” [1]
Endotoxins: “A pyrogenic product (e.g., lipopolysaccharide) present in the bacterial cell wall. Endotoxin can lead to reactions in patients receiving injections ranging from fever to death.” [1]
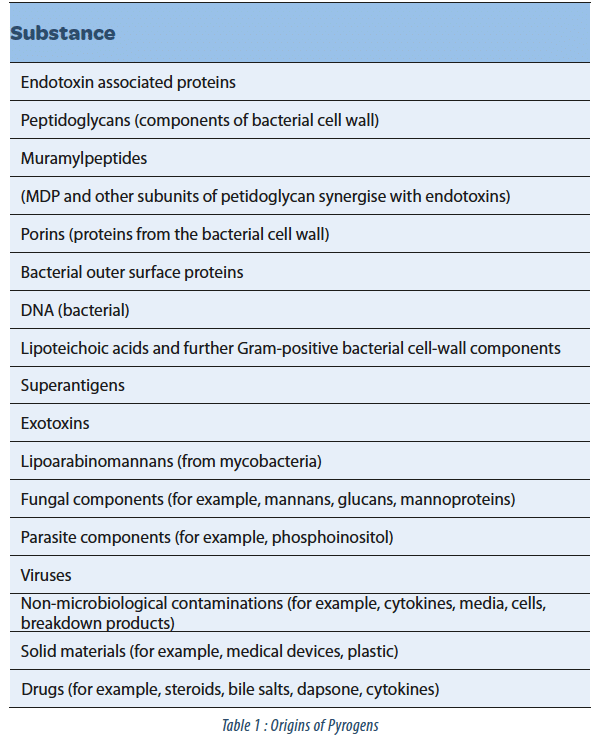
Lipopolysaccharides / endotoxins are a subtype of pyrogens originating from the cell wall of gram-negative bacteria that are both extremely thermostable and very potent when brought into contact with the human immune system. But they are by no means the only possible pyrogenic contamination – and the growing complexity of biotechnological products increased the risk of having pyrogens present that originate from a variety of microorganisms and other sources (see table 1).
History of pyrogen detection
The first mentions of pyrogenic substances date from the 1870s, but it was only in 1914 that pyrogen tests on rabbits were introduced in the British Pharmacopoeia. These tests are based on measuring the increase in body temperature of rabbits after injection of the product being tested.
Despite being a rather laborious in vivo assay and being specific for substances pyrogenic for the rabbit, the test was long the reference assay for detection of pyrogenic contaminations.
The first alternative developed in 1970 as a replacement of the pyrogen testing on rabbits was the test for bacterial endotoxin based on extracts of the blood of the horseshoe crab. This test was fast and reliable, but limited to the detection of endotoxin.
In order to have a full replacement of the pyrogen test on rabbits, a new test was introduced in 2000 that was based on the human immune system and its reaction to pyrogenic substances. The Monocyte activation test is able to detect all substances pyrogenic to humans and is expected to replace the rabbit pyrogen test in Europe from 2017.
Up to now, the authorities have accepted the endotoxin test as test for pyrogenic contaminations, but the growing complexity of the new products entering the market has caused a change in the harmonized European and American Pharmacopoeia chapter on pyrogen detection.
Acknowledging the growing risk of non-endotoxin pyrogens being present, a new prerequisite for the use of the endotoxin test is now a proof that endotoxins are the only possible contamination of a product or production process. Additionally, the phenomenon of Low Endotoxin Recovery needs to be investigated and the authorities call for strategies to overcome masking effects, whenever they are detected.
LER investigation – Hold-time studies
Investigations performed analyzing common protein formulation matrices have demonstrated that buffers containing citrate or phosphate in combination with surfactants like polysorbates are likely to cause LER. Although the mechanism is currently unclear, it has been suggested that the masking effect is due to a change in the structure of the LPS molecule, leading to a decreased binding to the pro-enzyme form of factor C that induces the enzymatic cascade used for detection of endotoxin in the Limulus amoebocyte lysate test (Reich et al., ). One of the many uncertainties today regarding the LER is the extend to which this masking also occurs in the human body, where recognition of the LPS depends on its binding to specific receptors on the surface of monocytes that in turn trigger the febrile response.
Although certain risk factors for the occurrence of an endotoxin masking have been identified, the only means of excluding a LER is currently to perform hold-time studies with endotoxin spiked directly into the sample[3]. The samples are then incubated at different temperatures, usually at 5 ± 3°C and 20 ± 5°C for up to 7 days. If a LER is present, the endotoxin shows a time and temperature-dependent decrease in concentrations, whereby the kinetic of the endotoxin masking depends strongly on the product, its formulation buffer and the source of endotoxin used. At Solvias, endotoxin hold-time studies are performed using the USP endotoxin standard, which corresponds to the expectations of the authorities, although testing with naturally occurring endotoxins may be more representative for the actual masking of real-life-contaminations.
Should a masking effect be observed, we suggest to run hold-time studies using the Monocyte activation test, as in some cases, the masking effect is specific for the endotoxin test and a change of test method is already enough to allow testing for pyrogenic contaminations. Endotoxin demasking agents are available, but development of demasking strategies is currently still difficult, as the mechanism underlying the masking is not quite clear. Changing the sample composition during demasking may also interfere with detection of non-endotoxin pyrogens, if the risk analysis has shown that such may be present and a Monocyte activation test or a pyrogen test on rabbits have to be performed.

Dr Anja FRITSCH – CONFARMA FRANCE SAS
Partager l’article
Références
[1] Guidance for Industry, Sterile Drug Products, Produced by Aseptic Processing — Current Good Manufacturing Practice FDA 2004
[2] ECVAM Workshop 43: novel pyrogen tests
[3] “Endotoxin recovery using limulus amebocyte Lysate (LAL) assay“.