Sommaire
- Une approche holistique de la maitrise de la contamination
- Part 2: Turning constraints into opportunities to accelerate Sterility Assurance performance
- Key Elements of a Successful Cleaning and Disinfection Program
- Améliorer la maîtrise de la contamination grâce à l’analyse de risque: un pilier de la stratégie CCS selon l’ICH Q9 et Q10
- The control of surfaces in cleanrooms: Questions & Answers
- Methods to Validate Disinfectants
- Clés du succès d’un projet de mise en place d’une solution de nettoyage GMP
- Nouvelle venue dans le monde de la désinfection ?
- Arrêté sècheresse : impacts & opportunités pour l'industrie pharma
CCS → Part 2. Turning constraints into opportunities to accelerate Sterility Assurance performance.
Dear readership of “la vague magazine”, as promised, I am coming back with this second edition to continue the CCS story.
These personal insights I have gained through my experience in the field of quality assurance and contamination control will give you a comprehensive and structured approach and guide you in your efforts to comply with GMP Annex 1 and, not only, because it will help you to be aware that regulatory compliance often perceived as a constraint, is a real accelerator of quality performance and particularly of sterility assurance performance.
Indeed, once the big picture of CCS is understood (explained in my previous article-part 1), your PQS embraced contamination control requirements, there are still a series of operational, organizational and strategic aspects that are essential to address before the benefits of a sustainable CCS system can be fully appreciated.
At the end of this article, I am giving some perspectives regarding a crucial up-coming strategic transformation of pharmaceutical industries making using contamination control data in day-to-day decisions possible, predicting and saving coming issues of contamination.
The following topics will be addressed:
- How to make CCS an efficient tool to drive performance?
- A sustainable CCS leads to an effective Inspection Readiness and Compliance Plan
- Insights into the coming strategic transformations of pharmaceutical industries to accelerate compliance with the GMP Annex 1 regulation
1. How to make CCS an efficient tool to drive performance?
In this part, I will focus on the importance of implementing a sustainable CCS, and how to achieve a level of maturity necessary for driving performance. Providing answers to the following questions will guide my analysis:
- What are the key elements to consider for achieving a sustainable and effective CCS?
- How to use CCS as a tool for driving performance in the pharmaceutical industry?
As already explained, CCS is coming reinforcing the PQS as a quality management system with a contamination control dimension. To be effective, CCS should be sustainable and embedded in the company’s organization. If this level of maturity of the PQS is achieved, quality and industrial performance are certainly guaranteed.
So, what are the key elements to consider for achieving a sustainable and effective CCS?
Let’s see together the three ” MUST HAVE ” prerequisites to be in place in the company and redesigned if necessary to facilitate CCS implementation and sustainability:
1.1 CCS Governance: Organizational Factor
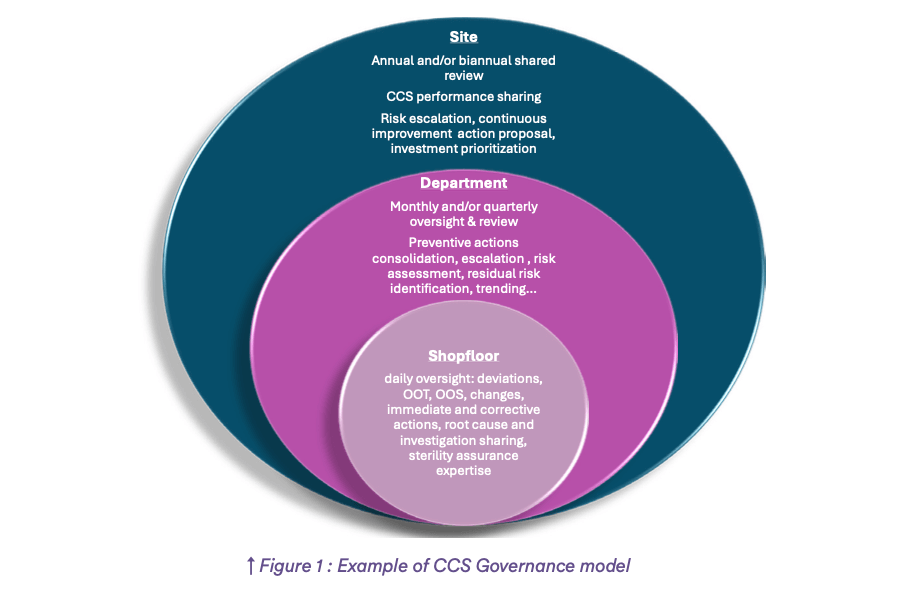
Clear roles and responsibilities regarding contamination control are a key element in the organization at all stages and during the entire lifecycle of the product (see part 1). For sterile products manufacturers, the existence of a well-orchestrated and efficient Sterility Assurance Governance with clear pathways for escalation is one of the most important tools to ensure CCS sustainability.
This Sterility Assurance Governance is crucial for driving CCS culture and awareness; risk culture related to contamination control and continuous improvement….
In order to make this organization efficient, it is essential to define key positions that require expertise in sterility assurance, sensitivity to quality culture and experience in quality management. People with influence skills will be helpful to drive changes and continuous improvement related to contamination control. Several levels of Sterility Assurance Governance are required depending on the size of the company and the number of production shopfloors and technologies, involving production, sterility assurance and operational quality as a minimum and other ad ’hoc stakeholders’ such as maintenance, QC lab and project leaders as appropriate.
It is important to involve production staff, including management, as key partners in contamination control. In this way, this governance will enable production to take ownership of contamination control and be aware of the associated risks for making the best decisions at the most appropriate time.
Sterility Assurance Governance with the right decision makers will ensure that: (Figure 1: Example of CCS Governance model)
- The entire process is under control
- The contamination risks are identified and managed using the PQS tools.
CCS performance and residual risks are escalated to the appropriate level of Governance for endorsement, mitigation action validation and prioritization of investment if necessary.
In your company, have you taken the time to evaluate whether existing governance structures meet the need for CCS sustainability?
It is therefore time to start thinking about your own organization and how to apply this guidance in order to define the best mechanism to put in place to facilitate the implementation of a sustainable Contamination Control Strategy.
1.2 CCS Anatomy : Organizational & Documentary Factors
The position of the CCS in the PQS and the mapping of its relationship with all the existing quality management processes in the company will clarify the organizational model and help to avoid duplicates and ambiguities.
Building a thoughtful and meaningful CCS is fundamental for the understanding of contamination issues and for the structuring and handling of these events in order to better understand the weakness of the actual strategy and to establish a permanent link with the CCS and how to update it
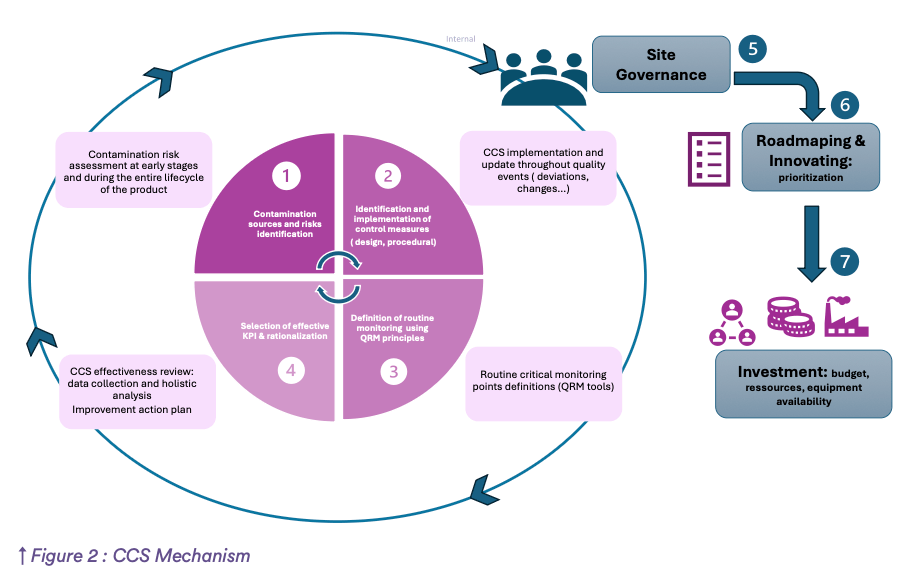
The structure of the CCS is very helpful for:
- The understanding of the strategy implemented in the company to prevent contamination:
- CCS mechanism (see Figure 2)
- CCS documents architecture
- CCS governance and responsibilities (see Figure 1)
- CCS-related quality & performance reviews (see Figure 3)
- Giving a simple and high-level explanation of every process (the grouping of similar processes in term of contamination control measures is possible) and making the link to existing documents (production instructions, validation documents….)
- Describing clearly the contamination control measures already in place (categorization by design, procedural, organizational and technical measures) to manage our identified risks
- Defining the adapted monitoring programs using QRM principles capable to detect any breach in contamination control (EM sampling program, maintenance program, Cleaning & Disinfection program, utilities sampling program…)
- Handling contamination investigations, impact assessment and identifying continual improvement pathways (CAPA)
As illustrated in Figure 2, the CCS mechanism of your company should be understood internally and easy to explain during inspections. This holistic overview shows that contamination control is a priority in your organization and will reinforce health authorities’ trust and brand image.
Ultimately, this ingenious anatomy of the entire CCS will ensure consistent performance by introducing a coming lifestyle transformation and why not a ” Paradigm Shift ” by acting before problems arise and stop wasting energy dealing with contamination issues with a short-term vision.
1.3 CCS performance review: Strategic factor
In your company, do you assess your CCS performance and take it into consideration in your global industrial performance assessment?
As you know, the actual GMP Annex 1 emphasizes the use of CCS for continuous improvement and performance optimization. The question then arises: how do you put this into practice?
Once your CCS is built, understood and its mechanism is smooth-running, it’s crucial to measure its effectiveness. The selection of appropriate metrics (measurable) issued from the existing routine monitoring programs imbedded in the PQS should be thought of and rationalized.
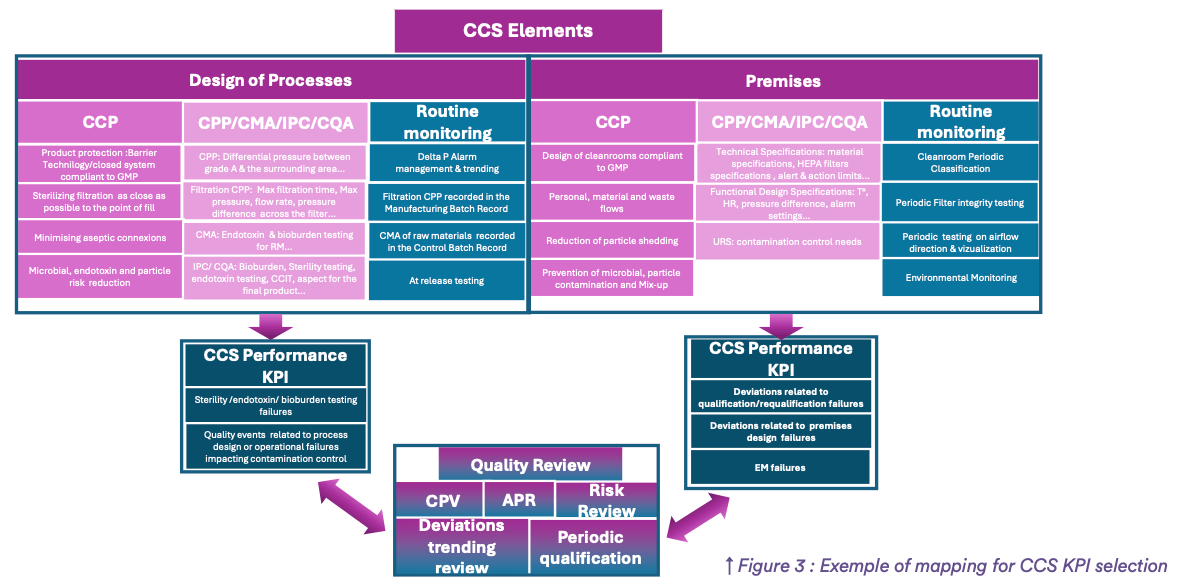
Ideally, every CCS element should be assessed with at least one metric to ensure at the end of the exercise that all CCS elements are under control. To be valuable, a tiny mapping of each CCS element should be performed as illustrated in the Figure 3 for “Design of the process” and “Premises” in the aim of identifying the CPP’s, CMA, IPC and CQA, the routine monitoring defined in the PQS and the most important indicator to measure the contamination state of control of the concerned CCS element.
In the example about: “Design of processes” in the figure 3, we can consider the percentage of failures of microbial testing to measure the overall CCS effectiveness during process steps. This indicator is reflecting the final result on the product of all the control measures in place. To complete this metric, it’s also valuable to consider the percentage and criticality of quality events (deviations, complaints, audit findings…) related to process design weakness or operational failures (leakage of the product, PUPSIT failures….) leading to unexpected production breaks. As Isolated incidents that are handled correctly may be harmless, repeated ones are often indicative of a loss of control and need to be treated with the highest interest.
In the example about: “Premises” in the figure 3, we can consider the percentage of EM excursions as this indicator is reflecting the final result on the environment of all the control measures that are in place. To complete this metric, it’s also valuable to consider the percentage of failures of requalification and reclassification exercises and the percentage and criticality of quality events related to design weakness of the premises.
I invite you to perform this exercise for all CCS elements to rationalize CCS KPI selection. Through this detailed mapping and analysis of these two concrete examples, we can define two levels of metrics:
CCS KPI Level 1: identification of weak signals predicting contamination that must not be ignored in order to achieve accurate and proactive CCS management.
CCS KPI Level 2: Statement of confirmed contamination breaches that only allow for a macro and reactive management of CCS.
Certainly, this in-depth mapping is time consuming but it’s crucial to select the key indicators of CCS performance ensuring proactive management in order to avoid critical events happening. These critical events are undeniably more time-consuming and lead undoubtedly to insufficient performance. Of course, the integration of these indicators in our routine monitoring and the design of an effective trending strategy are fundamental for achieving this goal.
2. How to use CCS as a tool for driving performance in the pharmaceutical industry?
It’s interesting to perform a CCS performance review on a regular basis to be able to measure the Sterility Assurance performance at any time and to make the appropriate decisions and orientations according to the trends and risks. To achieve this goal effectively, manufacturers should establish a structured and robust approach for data real time collection and consistent analysis in order to identify recurring patterns, anticipate potential problems and take proactive measures before product quality is affected. This is also highlighted in the EU GMP that emphasizes the importance of trending to maintain process stability and product quality (Sections 9 and 5.2 of EU GMP Annex 1)
Data collection and analysis are subjects of considerable interest. Indeed, how can we manage the big data generated in the running processes, understand it, categorize it, analyze it and, not least, correlate it to gain a thorough understanding of our process with the relevant strengths and weaknesses?
The undeniable solution for facilitating data processing is data digitalization. And not only that, once the digital data is available, it will need to be structured, key data will need to be tracked and the right connections between the different systems will also need to be established. At this time, interesting correlations between data, process performance and product quality outcome will need to be examined. I will leave you to presume that this is a full-value digital transformation project for the company, requiring thoughtful anticipation with very clear quality and business needs and objectives.
Once data is readily accessible and verified, we can leverage historical data to set CCS key performance indicator (KPI) targets and adopt proactive behavior and management.
Depending on the size of your company, this digital transformation will be more or less valuable and time-consuming. Consequently, the value of the service provided must be assessed. Let me give an example of a site producing one or two products in one shopfloor, it’s obvious that managing CCS performance review will be easier and can be centralized in the Annual Product Review for example. It needs some adjustments of the APR, but the exercise is easier, and digitalization is simpler. In contrast to a multi-product, multi-stage site, where the amount of data is much more significant and how it is analyzed depends on the stage of the process and many other factors. In this case, a well-designed digital solution is essential to facilitate the exercise and make it more effective and more time efficient.
Let’s return to GMP Annex 1 in section 3.1 that emphasizes that the PQS of manufacture’s should embrace the specific requirements of sterile product manufacture as discussed in part 1 of my article. The CCS & the PQS must work in harmony and the conclusion of the CCS performance review must be communicated to the top management on a regular basis and whenever necessary (see CCS Governance and the following highlighted requirement 3.1-part v) to oversee the state of control throughout the facility and throughout the product lifecycle and make the right decisions and strategical orientations related to contamination control management ( see Figure 2).
“3.1 The manufacture of sterile products is a complex activity that requires specific controls and measures to ensure the quality of products manufactured. Accordingly, the manufacturer’s PQS should encompass and address the specific requirements of sterile product manufacture and ensure that all activities are effectively controlled so that the risk of microbial, particulate and endotoxin/pyrogen contamination is minimized in sterile products. In addition to the PQS requirements detailed in Chapter 1 of the GMP guidelines (Part I – Basic Requirements for Medicinal Products), the PQS for sterile product manufacture should also ensure that:
1. An effective risk management system is integrated into all areas of the product life cycle with the aim of minimizing microbial contamination and to ensure the quality of sterile products manufactured.
2. The manufacturer has sufficient knowledge and expertise in relation to the products manufactured and the equipment, engineering and manufacturing methods employed that have an impact on product quality.
3. Root cause analysis of procedural, process or equipment failure is performed in such a way that the risk to product is correctly identified and understood so that suitable corrective and preventive actions (CAPA) are implemented.
4. Risk management is applied in the development and maintenance of the CCS, to identify, assess, reduce/eliminate (where applicable) and control contamination risks. Risk management should be documented and should include the rationale for decisions taken in relation to risk reduction and acceptance of residual risk.
5. Senior management should effectively oversee the state of control throughout the facility and product lifecycle. Risk management outcome should be reviewed regularly as part of on-going quality management, during change, in the event of a significant emerging problem, and during periodic product quality review. “
This requirement is interesting because it highlights that trending monitoring data outcome becomes meaningful when used to drive decision-making and to define preventive actions to maintain a Sterility Assurance state of control and compliance. Why not move towards a “paradigm shift”, switching from a reactive to a proactive decision-making process?
In summary, data analysis and digitalization are not a luxury, they are a fundamental necessity. It enhances business expertise. By combining scientific scrutiny, critical thinking, and digital tools, pharmaceutical professionals can address quality, productivity, and compliance challenges with peerless precision.
While organizational optimization, design correction solutions and investments in new technologies are undoubtedly costly and time-consuming, it is crucial to carefully consider their importance and priority to modernize our way of working to address deficiencies, avoid negative trends and recurrences and develop the company’s medium- and long-term strategic orientations.
3. A sustainable CCS leads to an effective Inspection Readiness and Compliance Plan
Implementing and maintaining an ingenious CCS by getting as close as possible to the proposed CCS model will undoubtedly strengthen:
- Awareness of residual risks and mitigation proposals
- Knowledge and expertise in processes
- Proactive approach when dealing with data and weak signals…
As a consequence of all the measures outlined above, the inspection readiness process is also strengthened, as by implementing a sustainable CCS, the company will achieve a high level of maturity in contamination control. The fundamental pillars are under control, and all these elements are integrated into a robust and sustainable quality management system. We can therefore talk about an effective and enhanced compliance and inspection readiness plan.
This clear and well articulated process will strengthen confidence of health authorities in our product quality, safety and innocuousness. The number, type and criticality of regulatory inspection findings is a strong indicator of the performance of the contamination control strategy.
CCS is once again leading to high-performance inspection readiness process that strengthens the company’s brand image and the confidence of patients and health authorities
4. Insights into the coming strategic transformations of pharmaceutical industries to accelerate compliance with the GMP Annex 1 regulation
The pharmaceutical industry is undergoing a silent but powerful revolution: the strategic use of data, every validation or production batch, every product testing, every quality event and every customer complaint generates a huge amount of data. But simply having data is not enough. What makes the difference? It is our ability to transform this data into intelligent decisions by using adapted digital tools that are able to transform data into value (Power BI, Minitab / JMP, Python, AI & Machine Learning…)
As underlined above, CCS data analysis is vital in the pharmaceutical industry because it helps to:
- Optimizing manufacturing processes: rapid detection of deviations, reduction of rejects and increased efficiency.
- Enhancing product quality: predictive analysis to anticipate non-conformities before they occur.
- – Accelerating quality investigations: Rapid identification of root causes of discrepancies and deviations to propose more targeted and more effective CAPAs.
- Controlling regulatory risks: Better analysis of trends (deviations, change controls, complaints, etc.) feeds into product quality reviews (PQR) and supports regulatory inspections.
- Promoting innovation: cross-referencing scientific and commercial data leads to more streamlined performance that is more focused on patients and market needs.
In this chapter, I would like to share some possible perspectives for pharmaceutical companies leading to a real digital and strategic transformation and why not break with the current paradigms, which focus on industrial performance in most cases and change them to quality and contamination control as the only way to achieve a good level of performance. This paradigm shift will encourage the adoption of a new mindset that prioritizes anticipation and learning from our experience and mistakes.
It certainly requires a transformation of our work tools and our way of working so the road ahead is still long… that’s why I speak about transformation which will take some years to be understood, prepared and implemented. The pharmaceutical industry must move forward and start shaping the future right now. And you, are your companies ready for this strategic transformation?
This coming strategic transformation is built fundamentally on:
- Digitalization & big data management and handling: moving from static PQS to dynamic PQS
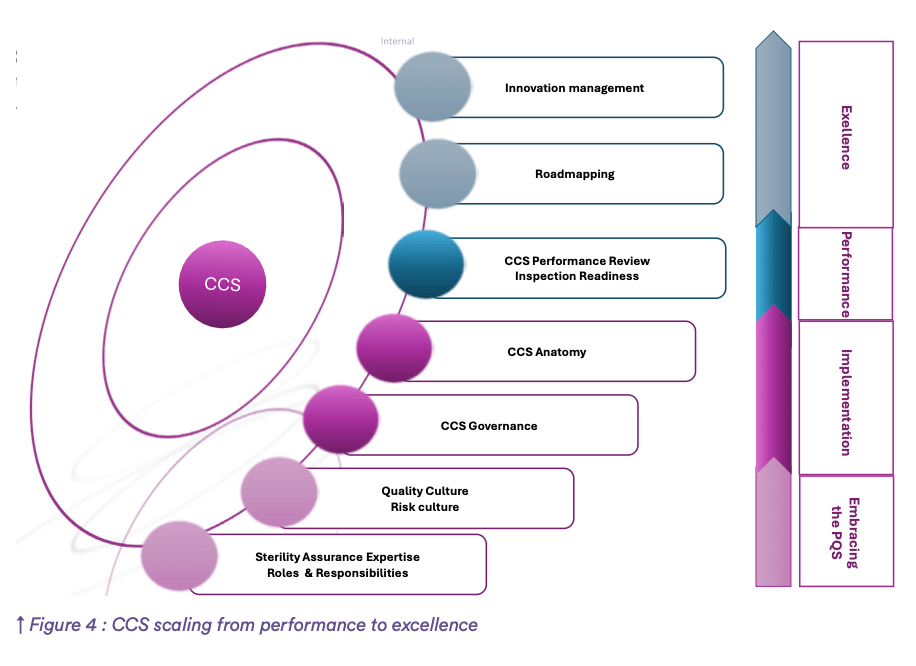
- Roadmapping & innovation management: CCS scaling from performance to excellence. (see Figure 4)
4.1 Digitalization & Big Data management and handling
The proposed revisions to Chapter 4 and Annex 11, as well as the introduction of Annex 22 in the EU GMP, mark a significant shift towards a digitally mature GMP framework and a dynamic PQS. It emphasizes that:
- Documentation must be controlled over and beyond paper
- Computerized systems are fundamental to quality, not optional
- Artificial intelligence, when permitted, must be governed like any other critical process
A new era of quality is coming….
Did you think about your PQS modernization? and the shift from static to dynamic Quality System?
This means that we are moving from validation (initial and periodic) to continuous routine monitoring and verification in real time and moving from initial fixed requirements to a dynamic model requirement embracing process and facilities/equipment lifecycle.
CCS data handling and management is once again concerned by digitalization effort of all the elements of the PQS individually and their consideration collectively to oversee the overall state of contamination control. I am sure that you all have emerging digital solutions projects in your companies, but I am convinced that these projects are handled individually according to silos organizations without establishing links between these different solutions in order to correlate data and use it to make strategic orientations and decisions.
To structure a dynamic PQS, it must be anticipated and mapped out in an ingenious manner. This requires collaborative work with IT, scientific and operational teams in order to design robust and traceable systems that comply with regulatory requirements. This major digital transformation project needs to be managed by high skills end to end project management professionals who will screen in detail every subtlety of the process and the associated critical control points to be managed.
The principle of the Work Breakdown Structure (WBS) or other project management tools will be useful for mapping out the project stages in detail, taking into account all the elements of the PQS, the associated quality reviews, the digital solutions used, the related critical control points and areas of required overlapping between these elements to establish solid relationships and pathways for continuous improvement and decision making.
This digital transformation must be guided by quality to ensure the use of trustworthy innovation tools allowing that technological progress translates into safe, effective and reliable results.
4.2 Roadmapping & innovation management
Once the CCS implementation phase is fulfilled, we enter in a phase of data analysis and correlation to measure the performance of the CCS and to offer the opportunity of continual progress and innovation. Once sufficient historical data is gained on our contamination state of control, we can switch from a “start-up” stage to a “scale up” stage of CCS implementation. So, we are in an improvement we are in an improvement phase of our CCS model by phase of our CCS model by introducing roadmapping and innovating management and finally being able to drive quality and production Excellence.
It’s obvious that these management tools are applicable for all organizations in all fields, but I am focusing on their applicability in the field of contamination control.
4.2.1 Roadmapping
As you know, many complex organizations have isolated silos of information. With data and knowledge locked away in various separate files and spreadsheets, it is practically impossible to make rapid, informed decisions. What is keeping these organizations from being agile, and why is this problem so common, even in leading companies?
One of the most effective strategies for addressing a lack of visibility within a company is to implement a comprehensive roadmap. It is vital for companies to undertake roadmaps and oversee them on a regular basis to maintain the flexibility necessary to continually deliver creative new offerings. Ideally, these regular roadmap exercises are digital and collaborative. The roadmap(s) should highlight links, such as new technological capabilities within the organization, and emphasize the cascading impact of business decisions over time. Multiple, digitally connected roadmaps are necessary to give decision-makers the necessary knowledge to see how decisions may impact other projects. Users can now compare multiple roadmaps to gain a holistic view of current projects and determine the feasibility and the prioritization of future projects. In fact, organizations can replace manual processes that compromise data accuracy with automated technology that consolidates data into a single, reliable source, facilitating access and collaboration.
Selecting and rationalizing the link between existing roadmaps such as: Sterility Assurance roadmap, compliance roadmap & digital and innovation roadmap is crucial for ensuring sustainability and reach CCS Excellence. So, let’s begin to build such empowering tools to make our priorities clear, visible and dynamic according to the company’s needs.
4.2.2 Innovation management
We spoke about innovation at several times… what does this word mean exactly? Innovation is the driving force for companies to stay relevant in the market.
The ability of organizations to innovate is recognized as a critical factor for their viability, competitiveness, resilience and renewal, and for the sustainable development of society. Adopting an innovation management system by an organization aims to improve its innovation performance (ISO 56001: 2024 innovation management system)
In the field of contamination control, promoting innovation is crucial for enhancing Sterility Assurance performance. The objective is to use high level innovative solutions to manufacture products, to monitor and trend data, to compile and correlate results and to plan and manage in a dynamic way the strategic projects of the company (see roadmapping chapter).
Regardless of the field or the activity of your company, innovation management needs to be embedded in the organization with clear roles and responsibilities. Let’s see together the main factors to consider to build Innovation management:
- Cultural factors: Embodying innovation within management (promoting courage and curiosity), embracing design thinking
- Organizational factors: investing in continuous learning, creating strategic partnerships, using agile methodologies, promoting cross-functional collaboration, developing a system for idea management, allocating dedicated resources, measuring and rewarding innovation
- Time factor: Prioritizing innovation
- Financial factor: Finding sources of funding
- Market uncertainty factor: Reducing uncertainty
A specific innovation roadmap is also useful to be combined with the whole panel of existing roadmaps in the company
Innovation in the field of contamination control is also crucial for sustainability, competitivity and business continuity.
Conclusion
After this analysis and compilation of the outcome of different management systems and tools referenced in quality standards and their established connection with the CCS. We can conclude that CCS revolutionizes the paradigm of pharmaceutical industry (see Figure 4) by:
- opening the opportunity for proactive thinking, proactive risk management and proactive decision making,
- embracing digital and innovative solutions and real time monitoring of the state of contamination control,
- using the outcome of CCS performance review to build strategic orientations,
- using digitally-connected roadmaps to empower midterm and long-term visibility and flexibility,
- embracing and promoting innovation management,
- switching from static PQS to an up to date and dynamic PQS.
So don’t hesitate to think about this coming big transformation and take benefit of its added value. PQS managing quality needs to be empowered to rationalize the existence of each of its elements and reinforce their collective impact to maintain patient confidence, product reliability, the integrity of an entire system and achieve Excellence.
To respond to future challenges and be up to date with the coming era of Quality….you can start to build an intelligent architecture of your needs in order to design an operational and ingenious GMP framework and anticipate these coming changes. The model that I speak about is somehow futurist, but technology is growing very fast, and the idea is to be prepared to face such a big transformation. So much is happening – don’t miss any of it!
- AI Artificial Intelligence
- APR Annual Product Review
- CAPA Corrective Action & Preventive Action
- CCP Critical Control Point
- CMA Critical Material Attribute
- CPP Critical Process Parameter
- CPV Continued Process Verification
- CQA Critical Quality Attribute
- EM Environmental Monitoring
- HEPA High Efficiency Particulate Air filter
- HR Humidity Relative
- IPC In Process Control
- KPI Key Performance Indicator
- PQR Product Quality Review
- PQS Pharmaceutical Quality System
- QC Quality Control
- QRM Quality Risk Management
- URS User Requirement Specification
Roadmapping: establishing a powerful strategic planning of strategic orientations of the company to enhance performance, innovation and sustainability. An effective roadmap creates a shared understanding of where a portfolio, product or project is going and why it’s going there. A roadmap is a visual, easy-to-understand representation of the series of planned events that need to happen to launch and grow a product or innovation or quality including sterility assurance performance.
References
- 1. GMP Annex 1
- 2. CCS A3P guide
- 3. CCS PDA guide (TR90)
- 4. ICH Q10 on Pharmaceutical Quality System
- 5. Innovation management system – Requirements (ISO 56001:2024)
- 6. ISO 56002:2019 Innovation management system – a practical guide
- 7. Draft of EU GMP Annex 22
- 8. Roadmapping Whitepaper-sopheon
Darine BEHLOUL