Sommaire
- Point de vue de la direction de l’inspection (DI) de l’ANSM sur le document ICH Q12
- ICH Q12 : les fondamentaux. Retours des travaux du GIC A3P ICH Q12
- First steps towards ICH Q12: Leveraging process understanding & development data to define process Established Conditions
- ICHQ12 Implementation from an Industry Perspective with a Focus on Established Conditions
- ICH Q12 compliance and Unified Quality and Regulatory Information Management
- Burkholderia cepacia a encore frappé
- Nouveau guideline Stérilisation de l’EMA
- How to store highly sensitive drugs? The benefit of functional coatings
How to store highly sensitive drugs? The benefit of functional coatings
Protecting medication from undesired interactions with the surrounding environment and preserving their efficacy during shelf life is one of the most pressing challenges for the pharmaceutical industry.
In particular, primary packaging material must adhere to stringent regulatory requirements as for instance stated by U.S regulations: “Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or adsorptive so as to alter the safety, identity strength, quality, or purity of the drug product beyond the official or other established requirements[1]”.
Type I borosilicate glass has been selected up to now as the most common packaging material. Indeed, containers made of this type of glass fulfill the requirements of the majority of the parenteral pharmaceutical products.
The pharmaceutical industry‘s difficult task and focus to overcome the early research and development phases of new pharmaceutical entities has led to an underestimation of the potential primary packaging/drug product formulation interactions. Although glass is a highly inert material, it must be evaluated as a complex container system whose properties have to be taken into consideration on the longer term. As an example, the storage of a small amount of water for injection in a Type I glass vial is a challenge as pH can shift overtime due to the potential container leaching elements into the liquid. It is of common knowledge that long term stability of small molecules entities based injected medicines can present technical challenges.
Nowadays, the pharmaceutical industry is more and more shifting towards the development of highly sensitive drugs, as for instance biologics, which are drugs typically derived from living organisms including therapeutic proteins, DNA vaccines, monoclonal antibodies, fusion proteins as well as gene and stem cell therapy[2].
With more than 200 billion USD sales in 2016 biologics already achieved ~20% of the sold pharmaceuticals including all routes of administration. The pharmaceutical market has been evaluated to grow with ~3-6% CAGR (in 2016-2021) whereas biologics have been significantly outgrowing the market with double digit CAGR (14 % in 2016). This trend seems to become more pronounced in the future[3].
To maintain the activity of highly sensitive drugs, the formulation needs to be properly adjusted to preserve the conformational integrity of the molecule and to protect functional groups from degradation.
For this purpose, additives such as buffers, salts, amino acids, sugars, and surfactants are typically used [4]. Special attention is needed to minimize the interactions between the drug and the formulation on one side and the drug and the glass surface on the other side.
Critical parameters for drug stability typically are the pH value of the formulation and the ionic strength. Extractable and leachable compounds from the glass can directly influence the formulation of newest drugs whose activity is sensitive to any changes of the storage conditions[5]. Protein adsorption on the glass surface is another important aspect to consider as it results in the loss of active compound, especially for highly diluted Active Pharmaceutical Ingredient (API)[6, 7, 8] but also conformational changes that can occur depending on the stability of the drug[9]. The changes can lead to formation of protein aggregates that might trigger immune response [10, 11].
The potential for phosphate buffer and ions leaching from glass vials is reported in several publications. The Swiss-based global contract manufacturer Legacy faced a similar situation when it came to manufacturing and storing a life-saving drug for an American pharmaceutical company. The API is registered under Investigational New Drug (IND) and is listed by the WHO as essential medicine. The product tended to interact with the vials, which led to particle formation, which occurred because of the formation of complexes from the phosphate buffer system and elements that leached from the glass. Results of stability studies showed an increasing amount of particles in the visible range within 1 to 3 months after manufacturing. Such particle formations are a serious problem; the affected products cannot be released to the market, because particles can lead to blood vessel obstruction in intravenously dispensed drugs and, in the worst case, cause a heart attack or stroke. This problem had to be solved to provide this life-saving drug to patients. For this reason, about two years ago, Legacy was looking for alternatives to the type 1 borosilicate glass used to date.
Therefore, this article will elaborate on how to store highly sensitive drugs.
Functional coatings
One way to adjust the properties of a pharmaceutical container to make it more suitable for biologics and their formulations is to apply functional coatings to its inner surface. This coating-process of the vial has been developed based on plasma enhanced chemical vapor deposition (PECVD) technology. Using a pulsed microwave source to ignite the plasma leads to a highly stable process with precise thickness control. This adaption of the PECVD technology is called PICVD (Plasma Impulse CVD) and has been used mainly for coatings in optical applications (halogen reflectors, halogen lamps, and eyeglasses)[12].
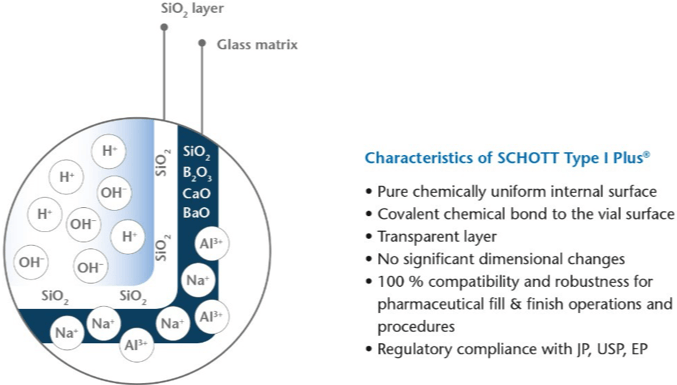
Today PICVD is a well-established technology to achieve functional coatings like SCHOTT Type I Plus® and SCHOTT TopLyo® used for sensitive biologics in liquid and lyophilized injectables for the pharmaceutical industry. With almost 100% material utilization Type I Plus® is free of HMDSO or siloxane like substances [14].
For Type I Plus® the thin quartz-like SiO2-Layer is covalently bonded making it extremely chemically durable, robust and 100% compatible for pharmaceutical fill & finish operations and procedures. The following figure 1 shows a schematic representation of a cross-section of Type I Plus® and a summary of its characteristics.
Enhancing vials by establishing an ion barrier
The thin SiO2 layer covalently bonded to the glass surface of Type I Plus® presents a unique diffusion-barrier for ions. This prevents leaching of ions from the glass and their interaction with the drug formulation. The efficiency of the barrier has been demonstrated under harsh conditions comparing the extracted elements of Type I Plus® vials with uncoated vials after autoclaving for 1 hour at 121°C with HCl-solution (0.1 mol/L) as shown in the following table1 [12]
The Type I Plus® test as release criteria
The values shown in table 1 for the uncoated container show typical expected extractable values under harsh conditions where Type I Plus® exhibits values, which are lower (with reduction factors between 15 – 350).
Borosilicate Type I container for parenteral applications need to fulfill the hydrolytic resistance test according to the Pharmacopeia. In order to prove the effectiveness of the ion- barrier even under harsh acidic conditions, the so-called Type I Plus® release test is used as shown in the following table 2 [15].
For the Type I plus® test, sodium has been selected as the representative element for product release, to determine the functionality of the diffusion barrier. It is one if the main has been set magnitudes lower than typical concentrations of leachables for uncoated vials, showing the effectiveness of the ion barrier.
Robustness of functional coated vials
To verify the influence of storage on the chemical durability of SiO2-layer, Type I Plus® vials have been compared with vials that have been stored for 11 years. The results showed that the storage had no influence on the chemical durability of Type I Plus® vials [16].
Further, depyrogenation at 380°C for 3 hours as worst case set up did not affect the functionality of the SiO2 – layer, underlining the robustness of the functional coating [16].
Further, numerous different stress conditions have been applied to Type I Plus® as described under WO 01/17569 A2. The stress test of the SiO2 – layer includes storage at extremely low temperatures for 6 weeks at -196°C after which the diffusion barrier properties remained intact [17].
Stability studies with functional coated vials
In order to evaluate if the vials are suitable for the antiviral drug that Legacy was manufacturing, the contract manufacturer conducted various stability studies. The tests also included an analysis on whether the conversion of the buffer from phosphate to citrate has an effect on the formation of particles. The studies lasted up to six months with further long- term stability tests currently ongoing.
The above study results from Legacy confirm previous analysis performed by SCHOTT. Indeed, the same result was also found when changing the buffer type, while the most favorable results were achieved by the combination of Type I Plus® as a container and converting the buffer to citrate. These studies encouraged Legacy to switch to Type I Plus® vials in the fall of 2019, advising the use of Type I Plus® to its customers to prevent any potential particle formation.
Avoiding the pH-shift to the highest extent possible
A pH shift describes the change of the pH from its initial value. There are numerous root causes described in literature for this phenomenon. However, they can all be traced back to two main contributors:
One is the dissolution of carbon dioxide from the headspace into the solution generating carbonic acid. This effect decreases the pH-value but can be rated as having a measurable but minor relevance.
The second effect with higher impact is the ion exchange process between the protons or hydronium ions in solutions with the sodium cation (Na+) on the surface of the glass container as shown in the following figure 2.
The ion exchange process removes protons from the solution. The equilibrium between protons and OH- -Ions is thereby shifted towards OH-, which makes the solution more basic. This ion exchange mechanism is predominant for acidic and neutral solution.
The extent of the ion exchange based pH-shift depends on the initial pH [18], on the fill volume [19] and also strongly on the amount of available sodium on the surface to be exchanged as shown by a series of test runs performed by SCHOTT-Rohrglas GmbH [20].
For instance, primary packaging material close but below the limit of the hydrolytic resistance value will have a pronounced pH-shift after autoclaving:
The containers were washed according to the ISO 4802 alkali release test, filled with distilled water, closed, autoclaved for 60 min at 121°C and then stored at room temperature. The sodium concentration, the pH value and the conductivity were measured after autoclaving, and then after 1 day, 1 week, 1 month, 6 month, 12 month and 18 month.
Figure 3 demonstrates the impact of high sodium concentrations on the surface of the container on ion exchange, conductivity and pH. The conductivity has been determined prior to autoclaving and was in accordance with the requirements for water for injection (WFI) with values equal or below 1.3 µS/cm at 25°C [21].
After autoclaving, the conductivity jumps to values above 6 µS/cm at 25°C. Further, the pH shifts from 5.5 to values to 7.5. Autoclaving accelerates the ion exchange process and indicates that uncoated vials fulfilling Type I requirements of the pharmacopeia can exhibit a pronounced shift in pH especially if the vial is filled below the nominal volume [22].
On the other side, the diffusion barrier of Type I Plus® has proved to fully avoid the ion exchange as demonstrated in the following figure
Figure 4 clearly shows that the SiO2 – layer acts as an ion barrier minimizing the conductivity shift and fully preventing the pH-shift- as well, even after autoclaving.
Applications of Type I Plus derived from minimizing the pH-Shift
The studies have shown that Type I Plus® is well established for the storage of WFI especially in case of longer shelf life (e.g. 5 years).
However, more and more Type I Plus ® is also being used due to the pH-stability for novel protein-based applications as for instance for rare diseases in the field of cell and gene therapy with highly sensitive proteins for blood factors or diseases of the central nervous system.
Besides denaturants [23], protein folding or unfolding of proteins can also be initiated by a pH-shift [24].
The pH sensitivity of protein conformity is well known and therefore buffers are being buffers also includes an additional variable to account for and in some cases a higher risk for glass delamination [25]. Even when buffers are used, Type I Plus ® ensures that the buffer capacity is maintained.
Proteins are very complex molecules that exist in a highly controlled environment, e.g. blood proteins are active in a very narrow pH range from 7.35-7.45 and barely active at other pH values [26].
Type I Plus® is therefore well established for commercial applications in the field of proteins for blood factors.
Applications of Type I Plus derived from minimizing leaching
Elements in Table 1 are typically leached out from the glass matrix of an uncoated vial made from FIOLAX® glass. Some of these elements may then interact with the drug formulation for instance, inducing the formation of a complex and thereby reducing the drug efficacy.
Furthermore, in case of light sensitive drugs FIOLAX® amber is the preferred choice of the pharma industry. Indeed its composition contains UV-absorbing additives as titanium and iron.
Especially for sensitive medicines it is therefore recommended to include PICVD coated FIOLAX® clear or amber vials in the initial screening study in order to select the most suitable container.
Aluminum
An increasingly attention is raised towards the interaction of the drug/formulation with aluminum in the field of parenteral nutrition.
Most adults ingest between 3 to 5 mg aluminum daily which is excreted through the kidneys and the urine. However, although aluminum is a body constituent itis toxic if ingested in higher amount. Chronic renal failure were the first symptoms reported caused by high aluminum intake. Aluminum is a contaminant in all parenteral nutrition solutions. Therefore, the FDA has published a code of federal regulations to define limits and procedures to prevent aluminum intoxication.
The maximum recommended intake of aluminum in parenteral preparations is 4-5g/kg per day.
The aluminum content for large volume parenteral (LVP) drugs used in total parenteral nutrition (TPN) therapy must not exceed 25 micrograms per liter [27].
Aluminum is a constituent of Type I glass and is added during its manufacture as aluminum oxide and will be leached into the product during its shelf life.
Several studies reported the presence of aluminum in parenteral nutrition due to storage in (uncoated) glass containers.
Bohrer, et al., determined the amount of leached out aluminum in amino acid containing parenteral nutrition. They tested 19 different amino acids and commercial nutrition formulation to investigate the effect of binding of amino acids from the leached out aluminum of the glass material. Contamination with aluminum was observed with cysteine, cystine, aspartic acid, and glutamic acid only. Leaching of aluminum from glass in the presence of amino acids mainly depends upon stability of the formed Al-amino acid complex i.e. the higher the stability of complexes the higher the stability of the amino acid to release aluminum [28]. For those cases standard Type I glass containers are not suitable as the amount of leached out aluminum will be too high. Instead, it is recommended to prevent the aluminum leaching by taking advantage of the ion barrier of Type I Plus®.
Applications of Type I Plus® derived from minimizing adsorption
Adsorption describes the phenomenon in which substances are physically bound to the surface of another material. Once the substance has formed a monolayer on the surface, it will not detach again. Adsorption is therefore an irreversible process.
Surface modifications by functional PICVD coatings like Type I Plus® are well established to reduce the adsorption of proteins on the surface. Because of the structural complexity of proteins, several factors need to be considered while defining the requirements of the packaging material.
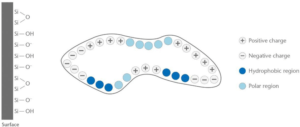
In general, proteins consist of hydrophilic (polar region), hydrophobic, positively charged and negatively charged regions. The distribution of share of those regions will determine the interaction with the glass surface.
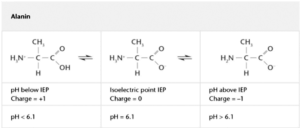
One of the most important aspect to understand the interaction of proteins with glass surfaces is the so-called iso-electrical point (IEP). Amino acids have the possibility to act as a hybrid ion which means combining a positively charged -NH3+-Group (protonated amino) with a deprotonated carboxyl group –COO-. This configuration has an overall neutral charge and within an electrical field the amino acid will move into the middle of the positive and the negative plate of a capacitor when performing electrophoresis. For pH-values below the IEP, the amino acid will be charged positively, for pH-values above the IEP the amino acid will be charged negatively. The principle is the same for all amino acids and proteins [29]. The nature of the amino acid or protein determines at which pH-value the IEP lies. An example is shown in table 2.
On the other side, the glass surface itself needs to be considered in detail as well. Uncoated Type I containers are hydrophilic, especially when they are freshly produced shown by a typical contact angle of 10-20°. Type I Plus® is considered as hydrophilic, however more hydrophobic as an uncoated Type I container, also shown by a reduced fogging tendency compared with an uncoated containers within a lyophilization process [30].
Mathes, et al., investigated the impact of formulation parameters as pH and ionic strength on the Immunoglobulin adsorption on borosilicate glass. For pH-values in the area of the IEP of the protein, hydrophobic interactions could occur whereas for pH values below the protein IEP, electrostatic interactions are becoming more dominant [31].
Therefore, for pH-values around the IEP of the protein hydrophilic proteins will therefore adsorb less on a Type I Plus® glass surface.
However, for pH-values significantly below the IEP the protein will exhibit an overall positive charge, which could lead to a pronounced adsorption of the protein on an uncoated borosilicate glass surface. The nature of an uncoated Type I glass is predominantly negatively charged as shown in figure 6.
Pronounced adsorption
The PICVD process as applied for Type I Plus® is a plasma reaction, where an activated SiO+ formed from the precursor gas Hexamethyldisiloxane diffuses to the nearest surface forming a SiO2-Layer [12].
Type I Plus® might perform better in cases where uncoated vials exhibit a pronounced adsorption of the drug on the inner surface.
This is also in accordance with the results reported by Doran where the adsorption of protein to glass was minimized by the use of a siloxane coating and the addition of surfactant. [32]
Schwarzenbach, et al., studied the adsorption and adsorption of interferon alpha-2a on uncoated and coated Type glass and mica surface. Atomic force microscopy was used to measure directly the adhesion forces between interferon molecules and the inner surface of the vials under aqueous buffered conditions. The authors demonstrated that the adhesion force of Type I Plus® was reduced by 40% compared with the uncoated container [33].
Chi reported that the adsorption of proteins to glass could be reduced by choosing a solution pH at which both the protein and the glass had a net negative charge. Therefore, choosing a pH significantly above the IEP will reduce the amount of adsorbed protein on borosilicate glass [34].
Apart from the adjustment of the pH of the formulation and the adjustment of the inner surface of the container, further conventional ways to reduce the adsorption of the therapeutic protein include the use of surfactants like polysorbate 20 or 80 [13]. Further, different amino acid buffers are well established as stabilizers like Histidine, Methionine, Glycine and Arginine. Histidine has been reported to provide maximal stability and is able to reduce protein aggregation [35].
Especially amino acid with an IEP at alkaline pH-values like Arginine (IEP: 11.1), Lysine (IEP: 9.6) and Histidine (IEP: 7.6) will be positively charged in a wide pH-range and can be considered as sacrificing proteins adsorbing on a negatively charged uncoated borosilicate glass container. This is in line with the capability reported of positively charged amino acids to particularly enhance the stability of protein formulations and suppress aggregation [36, 37].
Conclusion
The pharmaceutical industry is increasingly developing biologics, which are drugs typically derived from living organisms. Many of these drugs/formulations are highly sensitive and are prone to interact with their environment. Subsequently, this increases the requirements for primary packaging. The aim is to minimize the interaction between the drug/formulation and the primary packaging container such as adsorption of the drug on the inner surface and ion leaching. Further, it is essential to maintain the conditions and interactions of the drugs/formulation stable during shelf life. The stability of the drug is influenced by numerous factors, e.g. the pH value and the ionic strength. High concentrations of leached out ions is followed by an ion exchange altering the pH value, which reduces the stability and thereby the activity of the drug.
Functional coatings on the inside of the pharmaceutical packaging can reduce these effects. One example is Type I Plus vial, which has a SiO2 layer that acts as an ion barrier. This offers the advantage to prevent leaching of glass “elements” and to minimize the risk of interaction with the drug formulation, enhancing the shelf life of the drug.
Further, it has been shown that the SiO2 layer of functional coated vials might adsorb much less of the drug product compared with an uncoated container. Therefore, it is recommended to include functional coatings into the stability study for highly sensitive drugs in general.
Even though, functional coatings are more and more being established for biologics, they also offer the opportunity for smaller traditionally produced molecules (chemicals) to avoid a fundamental change of the drug/formulation.
Partager l’article
Bernhard Hladik – SCHOTT AG
Bastian Kloefer – LEGACY PHARMACEUTICALS SWITZERLAND GMBH
References
1.Heinl, C., Extractables Profile of Aluminosilicate Glass Prior to Chemical Treatments, PDA Letter Volume LIII. Issue 9, October 2017
2.AMGEN, An Introduction to Biologics and Biosimilars, MC52904, 2011
3.Tomasz Kluszczynski, IQVIA™, 2018 Global Trends & Local Dynamics
4.V. Gervasi, R. Dall Agnol, S. Cullen, T. McCoy, S. Vucen, A. Crean,, Parenteral protein formulations: An overview of approved products within the European Union, European Journal of Pharmaceutics and Biopharmaceutics 131 (2018) 8-24
5.Claudia Dietrich, Florian Maurer and Holger Roehl, Wolfgang Frieß, Pharmaceutical Packaging for Lyophilization Applications, Freeze Drying/Lyophilization of Pharmaceutical and Biological Products, Third Edition, 2010
6.Song D, Hsu LF, Au JL, Binding of taxol to plastic and glass containers and protein under in vitro conditions, Journal of Pharmaceutical Science 1996, 85:29-31
7.Grohganz H, Rischer M, Brand M, Adsorption of the decapeptide cetrorelix depends both on the composition of dissolution medium and the type of solid surface, Eur J. Pharm Sci 2004, 21:191-196
8.Volkin DB, Middaugh CR, Formulation, Characterization and Stability of Protein drugs, New York: Plenum Press 1996: 181-217
9.Norde W, The behavior of proteins at interfaces, with special attention to the role of the structure stability of the protein molecule, Clin Mat 1992, 11:85-91
10.Rosenberg AS 2006, Effects of protein aggregates. An immunologic perspective, AAPS J 8(3): E501-E507
11.Schellkens H 2002 Immunogenicity of therapeutic proteins: Clinical Implications and future prospects. Clin. Ther 24 (11): 1720-1740
12.Marten Walther, Volker Rupertus, Christina Seemann, Jutta Brecht, Robert Hormes, Robert W. Swift, Pharmaceutical Vials with Extremely High Chemical Inertness, PDA Journal of Pharmaceutical Science and Technology, Vol.56, No. 3, May/June 2002
13.Jared S. Bee, Theodore W. Randolph, John F. Carpenter, Steven M. Bishop, Matiana
N. Dimitrova, Effects of Surface and Leachables on the Stability of Biopharmaceuticals, Journal of Pharmaceutical Science, Vol. 100, No. 10, October 2011
14.Extractable and leachable study with SCHOTT Type I Plus® vials performed with SCHOTT Pharma Service. Analysis Report No 11-2012-00327. See summary of the results in the Addendum
15.SCHOTT Standard operation procedure (SOP) QAA00901 for the test for hydrolytic resistance of the inner surface of Type I Plus
16.Volker Rupertus, SCHOTT statement (controlled document): Chemical durability after 11 years storage time and 3 hours retention time at 380°C for SCHOTT Type I plus® glass containers, 12th of December 2013
17.Improved container composition for radiopharmaceutical agents, WO 01/17569 A2, publication date: 15.03.2001
18.Rehm, K. Evaluating the Surface Resistance of Ampoules with the Aid of pH- Determinations. Drugs made in Germany, 1967, Vol.10, pp. 37-42
19.Kucko, N. W.; Keenan T.; Coughlan A.; Hall M.M., Fill Volume as an Indicator of Surface Heterogeneity in Glass Vials for Parenteral Packaging, J. Pharm. Sci. 2013, 102, 1690-1695
20.Michael Rössler, SCHOTT-Rohrglas GmbH, Newsletter, Pharma Information Letter, Extractables, 11th Edition, 07/2005
21.USP 40, Physical Tests / <645> Water conductivity
22.Bernhard Hladik, Florence Buscke, Robert Frost, Uwe Rothhaar, Comparative Leachable Study for Glass Vials to Demonstrate the Impact of Low Fill Volume, PDA Journal of Pharmaceutical Science and Technology, Vol. 73, No. 4, July-August 2019
23.Edward P. O’Brien, Bernard R. Brooks and D. Thirumalai, Effects of pH on proteins: Predictions for ensemble and single molecule pulling experiments, J Am Chem Soc. 2012 Jan 18; 134(2): 979–987.
24.Whitten ST, Wooll JO, Razeghifard R, Garciá.Moreno E B, Hilser VJ, The origin of pH dependent changes in m-values for the denaturant-induced unfolding of proteins, J Mol Biol. 2001 Jun 22, 309(5): 1165-75
25.UWE ROTHHAAR, MICHAELA KLAUSE , BERNHARD HLADIK, Comparative Delamination Study To Demonstrate The Impact Of Container Quality And Nature Of Buffer System, PDA J Pharm Sci and Tech 2016, 70 560-567
26.Bettine Boltres, When Glass meets Pharma, Editio Cantor Verlag, ISBN 978-3-87193- 432-2, 2015, Page 61
27.FDA, CFR – Code of Federal Regulations Title 21, Food and Drugs, Sec. 201.323: Aluminum in large and small volume parenterals used in total parenteral nutrition
28.Bohrer, D., do Mascimento, P. C., Binotto, R. Carlesso, R. J. Trace Elem. Med. Biol. 2001, 15, 103-108
29.David Whitford, Proteins – Structure and Function, John Wiley & sons Ltd., 2005
30.Min Hunag, Ethan Childs, Krik Roffi, Fawziya Karim, Jennifer Juneau, Bakul Bhatnagar, Serguei Tchessalov, Investigation of Fogging Behavior in a lyophilized drug product, Journal of Pharmaceutical Science, 2018
31.Mathes, J., Friess, W., Eur. J. Pharm. Biopharm. 2011, 78, 239-247
32.Doran PM. 2006. Loss of secreted antibody from transgenic plant tissue cultures due to surface adsorption. J Biotechnol 122(1): 39-54
33.Schwarzenbach, MS. Reimann, P., Thommen, V., Hegner, M., Mumenthaler, M., J., et al., PDA J. Pharm. Sci. Technol. 2002, 56, 78-89
34.Chi EY, Weickmann J, Carpenter JF, Manning MC, Randolph TW. 2005. Heterogeneous nucleation-controlled particulate formation of recombinant human platelet-activating factor acetylhydrolase in pharmaceutical formulation. J Pharm Sci 94(2): 256-274
35.H. Santana, Y. González, P.T. Campana, J. Noda, O. Amarantes, R. Itri, et al., Sceening for stability and compatibility conditions of recombinant human epidermal growth factor for parenteral formulations: effect of pH, buffers, and excipients, Int. J. Pharm. 452 (1) (2013) 52-62
36.K.M. Forney-Stevens, R.H. Bogner, M.J. Pikal, Addition of amino acids to further stabilize lyophilized sucrose-based protein formulations: I. screening of 15 amino acids in tow model proteins, J. Pharm. Sci. 105 (2) (2016) 697-704
37.V. Gervasi, R. Dall Agnol, S. Cullen, T. Mc Coy, s. Vucen, A. Crean, Parenteral protein formulations. An Overview of approved products within the European Union, European Journal of Pharmaceutics and Biopharmaceutics 131 (2018) 8-24