Sommaire
- Annex1 & CCS → How to leverage mathematics & IT tools for driving improvement?
- CCS → Part 1. Deep dive on CCS understanding
- No detail left behind: A holistic approach to opening authorization in Sterile Manufacturing
- Production of incretin mimetics → Weight under control
- The next generation of reagents for Endotoxin Testing for the next generation of scientists in the pharmaceutical / medical device industry
- Le secret des produits pharmaceutiques et cosmétiques réussis, de la formulation à l'application
- A Toolbox for an Effective Technology Transfer
- Excess heat in pharmaceutical industry
The next generation of reagents for Endotoxin Testing for the next generation of scientists in the pharmaceutical / medical device industry
The pharmaceutical industry—an exciting, fast-paced world where innovation changes the quality of human life. If you’re a recent graduate stepping into this field, you are a part of a new generation poised to shake things up and make a difference. One topic where your fresh perspective is truly needed? Endotoxin testing. This critical process keeps patients safe because it ensures that injectable drugs and medical devices are free not only from viable harmful bacteria (subject to sterility testing) but also free from their ghost components – endotoxins.

Finished product testing supported by well-designed process control helps ensure the quality and safety of given sterile pharmaceuticals, from small molecules to complex biologicals and combination products (1).
Testing for bacterial endotoxins has been an integral part of the process control for half a century (and prior to that as an in-vivo pyrogen test done in live rabbits since 1942)(2). Starting in the 1980s, the use of live rabbits had been largely replaced by the Limulus Amebocyte Lysate (LAL) test, an in-vitro test. Since then, the LAL test has been the gold standard for endotoxin testing around the world (3, 4)! Saving millions of rabbits and humans. But here’s the catch—the LAL test is completely dependent on a natural resource derived from the blood of wild marine arthropods – the horseshoe crab (5).
With the support of regulatory bodies, technology, and a strong push by investors, the industry has now started moving in a new, “greener” direction (6, 7, 8). One that you can help shape! Let’s talk about how the next generation of reagents for endotoxin testing is more than just science—it’s about sustainability, secure supply chain, innovation, and making a difference.
Why Endotoxin Testing Matters
Imagine this—you’ve created a groundbreaking new drug. It has passed rigorous trials and is set to help millions. But before it hits the market, it must be tested for endotoxins—tiny, invisible but toxic components of the cell envelope of Gram-negative bacteria. Endotoxins, if in abundance, circulating in our circulatory system or cerebrospinal fluid, can trigger severe, or even lethal reactions in patients. That’s why it’s a huge part of ensuring pharmaceutical product safety. Testing for bacterial endotoxins isn’t just an industry standard – it’s the law and a life-saving measure.
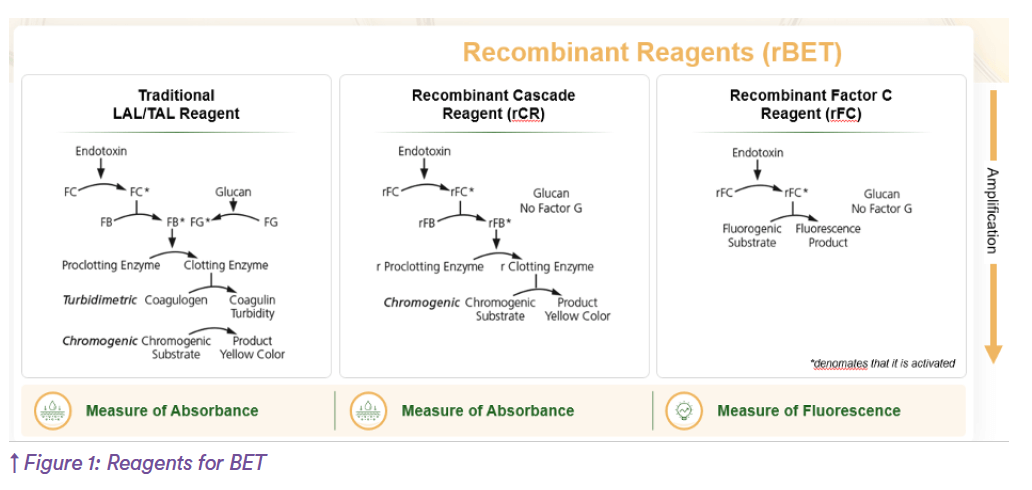
The first reagent for the bacterial endotoxins test was developed by the scientists who formed Associates of Cape Cod, Inc. (ACC) in the 1970s based on the discovery and research performed by Dr. Fredrick Bang and Dr. Jack Levin at the Marine Biological Laboratory (now University of Chicago) in Woods Hole, MA, USA(9). The raw material for all LAL reagents is blue blood (aka hemolymph) donated by the horseshoe crab. The blood clotting cascade present within amebocyte’s granules detects endotoxins (regardless of the bacterial strain) with an extremely high sensitivity and specificity.
The Biodiversity Challenge
Horseshoe crabs play a key role in coastal ecosystems and have done so for eons, surviving 5 mass extinctions and a meteor impact(5). Their eggs are also a critical food source for migrating birds, and they have been part of the oceanic food chain for millions of years. While their collection and release for LAL production is highly regulated along the eastern seaboard of the United States, protecting the ecosystems and reducing the need of their blood cells is a responsibility of the LAL and pharmaceutical / medical device industry and regulators worldwide(10). Reducing and/or eliminating the use of wild animals is a common and worthy goal across all borders. For the new generation of pharma professionals, this is where you can make a difference (11).
Innovating for Sustainability
- The future of endotoxin testing is innovation, and it’s happening in ways that honor both science and sustainability. Meet the recombinant reagents for endotoxin testing (rBET): Recombinant Factor C (rFC) and Recombinant Cascade Reagents (rCR). Thanks to biotechnology that identified and cloned the key endotoxin-detecting proteins, they are now expressed in bioreactors without ever using a drop of horseshoe crab blood ever again(12, 13, 14, 15)! Here’s why recombinant reagents are a game-changer:
– Ethical and Sustainable: It supports the pharmaceutical industry’s shift toward ethical practices by minimizing the impact on wild animals (16). - Innovative bringing improved reproducibility and specificity to endotoxins: rBET reagents are highly characterized, therefore their performance is highly reproducible, with no cross-reactivity with 1,3-beta glucans (unlike LAL reagents)(13).
- Ready for automation: rBET reagents are compatible with various liquid handling systems, depending on the needs of the lab(7).
The best part? Global regulatory bodies are not only increasingly accepting rBET as an alternative to LAL testing but strongly encouraging pharma to implement their use (17, 18, 19)
Supply Chain Resilience Starts with Innovation
Beyond protecting crabs and ecosystems, these advancements also strengthen global supply chain of these critical reagents(16). Think about it dependance on a single source of raw material (Limulus polyphemus, the north Atlantic horseshoe crab) to make most globally used LAL reagents in the US may introduce some risk. The supply chain could potentially be vulnerable to shortages, delays, and regional shipping disruptions. Secondly, all the raw material for LAL must be collected during a short season (which continues to shrink due to locally imposed restrictions). This can be further impacted by changing weather patterns and rising sea levels. Compare that to rBET grown in a bioreactor, which can be made on different continents, can be scaled up or down as needed and has no dependency on access to the horseshoe crabs. By adopting rBET reagents, pharmaceutical companies create a more resilient, reliable supply chain that’s better equipped to handle global stressors (20).
Sustainability and resilience are no longer buzzwords—they’re necessities. And as someone new to the field, you can help push them from concept to reality just like Thomas Edison.

Become an rBET Influencer
You might be thinking, “What can I do as someone just starting out?” The truth is, your voice and values matter more than they seem.
1.Stay Educated: Keep learning about new advancements in pharmaceutical science. Technology moves fast, and staying informed ensures you’re ready to lead the way(21, 22).
2.Ask Questions: Get curious about the sustainability practices of your workplace. How are they adopting modern, ethical testing methods like rBET reagents? What steps are they taking to reduce their ecological impact?
3.Learn From the Pioneers in the Industry: Read sustainability reports of other companies, research and interview their employees on how the other companies work towards sustainability (23).
4.Champion Change: Advocate for innovations that prioritize both patient safety and environmental stewardship. If your company isn’t using modern testing methods, speak up and suggest it (24).
5.Collaborate for Biodiversity: Partner with conservation organizations or support research into sustainable practices. Aligning the goals of pharma and environmental science can lead to incredible breakthroughs. (5)
A Future You Help Shape
Every generation is known for demanding “better”— better ethics, better practices, and a better future – than your parent’s or grandparent’s reality. Your entry into the pharmaceutical world comes at a pivotal moment in healthcare history, as growing human population puts increasing demand on the industry and resources, technology and innovation step in to help answer the call. Endotoxin testing is just one piece of the puzzle, but it’s a critical one. By pushing for more sustainable solutions and supporting biodiversity, you’re doing more than contributing to safe medicine. You’re making a statement—that science and nature can thrive together in harmony. And in doing so, you’ll leave a legacy you can be proud of.
Welcome to the new age in pharma, where resilience and sustainability are more than goals—they’re the foundation of everything we do. Now, it’s your turn to take the baton and help lead this change. Will you rise to the challenge?
Partager l’article





