1 year after the implementation of Annex 1
Forum A3P Spain
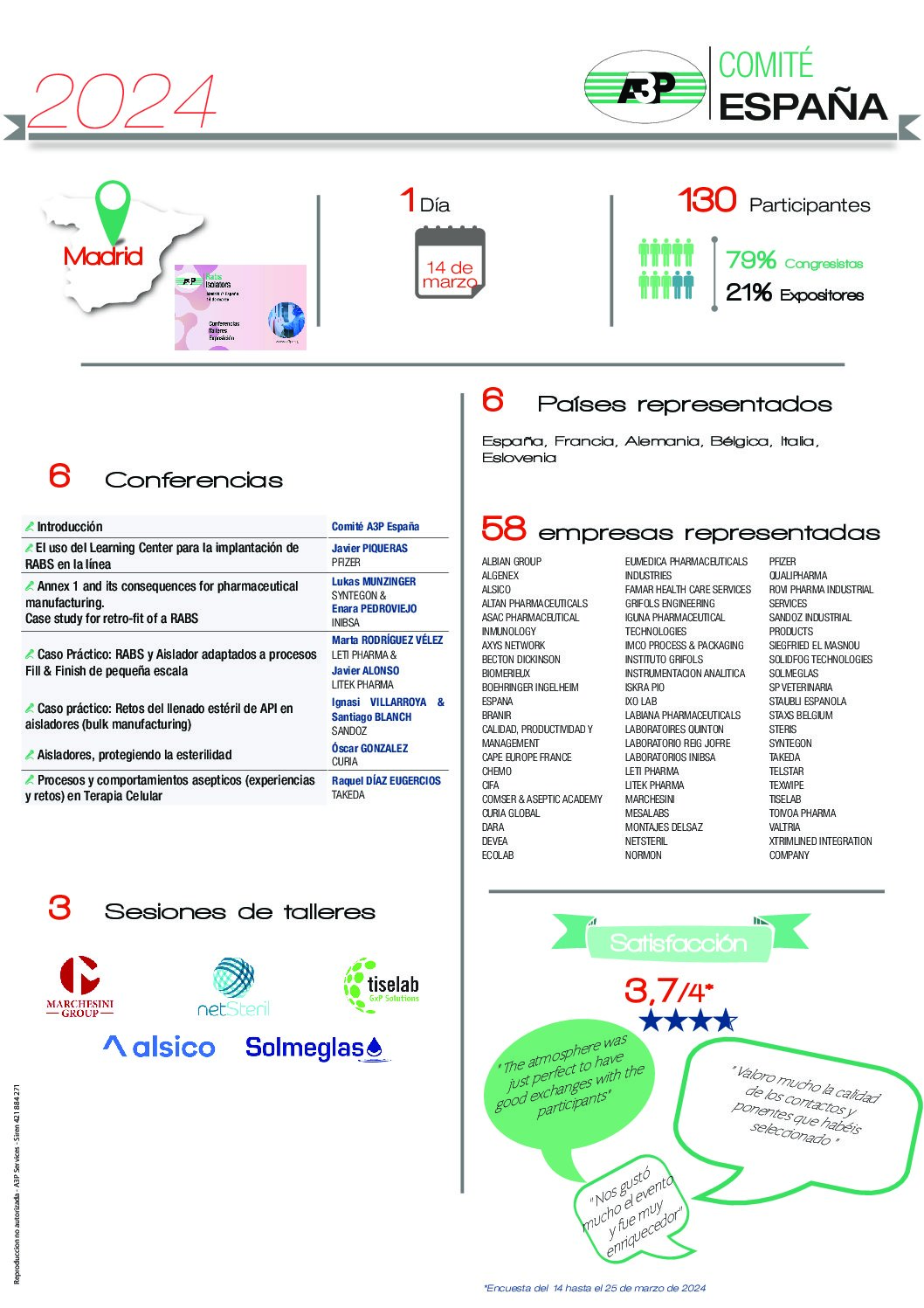
VENUE
Hotel SB Diagonal Zero, Barcelona
DATE
November 14th, 2024
Save The Date !

FORMAT
Conferences, Round Table, Workshops, Exhibition
SHARE
The new Annex 1 published on August 25, 2023, is focused on reducing the risks of product contamination in the manufacturing processes of a sterile product, especially when it is an aseptic process and not terminal sterilization. It includes regulatory updates, changes in other chapters of the GMP, use of tools described in the ICH Q9 and Q10 guidelines, aims to eliminate ambiguities and considers advances in new technologies.
The conference will address challenges, learning and experience gained one year after the implementation of Annex 1.
Recognized experts in their field will share their experience and knowledge and give details on what difficulties they have encountered in implementing the new requirements and how they have dealt with them. At the end of the day, you will be able to exchange your concerns and ideas with industry professionals during a round table to better understand the problems and innovative solutions. In this round table, the problems raised by the attendees will be discussed anonymously (attendees are encouraged to propose, beforehand or during the day itself, the issues they would like to be addressed) and the most appropriate options for their resolution will be debated.
🎧 Simultaneous translation service 🎧 : Spanish <-> English
Thursday 14 November

Partners Sessions
Session 1 – 12:00
Session 2 – 13:00
Session 3 – 15:45
Map of the exhibition
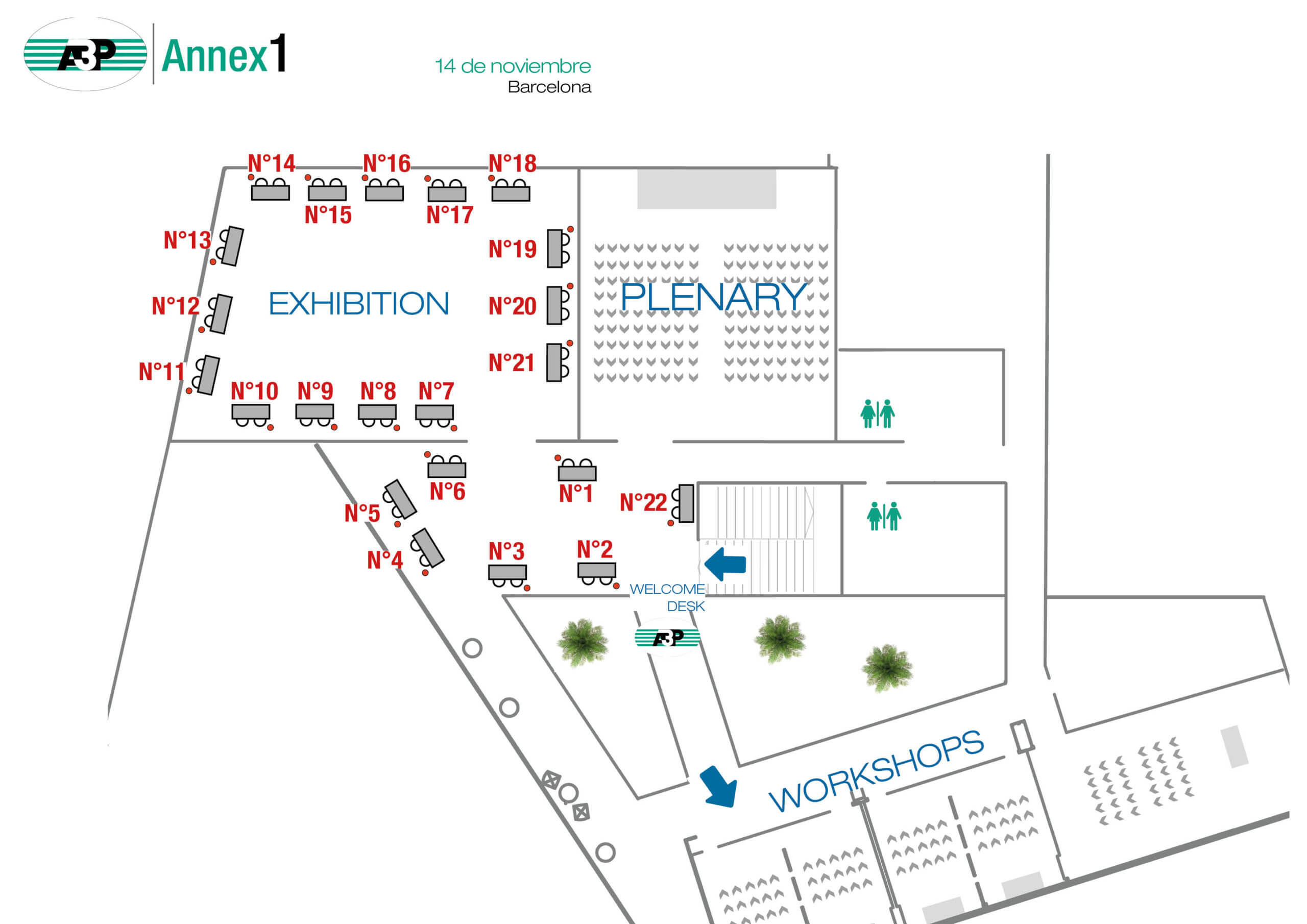
| Company | N° Stand | Company | N° Stand | |
| MARCHESINI | 1 | STERIS | 12 | |
| ELIS CLEANROOM | 2 | CONTEC | 13 | |
| QUALIPHARMA | 3 | CHARLES RIVER | 14 | |
| NOVATEK INTERNATIONAL | 4 | LITEK PHARMA | 15 | |
| OPTIMA | 5 | MESALABS | 16 | |
| COMSER & ASEPTIC ACADEMY | 6 | VESTILAB | 17 | |
| NETSTERIL | 7 | BECTON DICKINSON | 18 | |
| BIOMÉRIEUX | 8 | REDDITCH MEDICAL | 19 | |
| CYTIVA | 9 | STAXS | 20 | |
| IZASA SCIENTIFIC | 10 | TISELAB | 21 | |
| INSTRUMENTACIÓN ANALÍTICA | 11 | ALBIAN GROUP | 22 |
Services included with the Table Top stand
- The participation of 1 person
- A dedicated exhibition area where you can install your products and communication tools: umbrella stand, roll-up, display, product, …
A basic equipment including a 180×80 cm table, 2 chairs and 1 electrical connection (2kw) without circuit breaker - Breaks and meals as indicated in the program
- Illimited Wifi
- A badge that gives you free access to the entire technical and scientific programme.
- The list of participants to the event

Registration form
Participate in A3P event 1 year after implementation of Annex 1
Registration fee: 500€ without VAT (VAT 20%)
CONTACT
Sébastien BOYER:
Phone number: +33 (0) 4 37 28 30 51
Email: sboyer@a3pservices.com
Hotel SB Diagonal Zero
Address: Plaza de Llevant, s/n, Sant Martí, 08019 Barcelona
VENUE