Sommaire
- La restriction des PFAS dans l’industrie : enjeux réglementaires et impacts sur l’industrie pharmaceutique
- La technologie Blow-Fill-Seal dans l’industrie pharmaceutique : performance, applications et durabilité
- Key Allies in Preventing Contaminants and Impurities in Bioproduction
- Choosing the right vial: packaging sterile drug products with foresight
- Combination Products in the United States and European Union: Differences and proposed strategy to prepare common CTD Quality Module 3
- Blood plasma processing. When every drop counts
- L’analyse de la normalité en Continued Process Verification
- Qualification of impurities
- Pharma 2052
Key Allies in Preventing Contaminants and Impurities in Bioproduction
In the biopharmaceutical industry, the prevention of microbial contaminants and the control of process related impurities are critical requirements to ensure patient safety, therapeutic efficacy, and regulatory compliance. As processes become more complex and regulatory expectations intensify, Blow-Fill-Seal (BFS) and Single-Use Systems (SUS) technologies are emerging as key solutions to strengthen risk management. By minimizing potential entry points for contaminants and streamlining production flows, these innovations address the industry’s demands for robustness, traceability, and responsiveness.

Indeed, contamination of a production batch can not only compromise drug quality but also result in significant economic losses. BFS and SUS technologies provide advanced technical solutions that significantly reduce both microbiological and chemical risks throughout the manufacturing cycle. Mastering these risks is essential to ensure continuity of industrial operations, protect corporate reputation, and meet increasingly stringent global health authority requirements.
The digitalization of processes, the integration of connected sensors, and the rise of data management in BFS and SUS systems further enhance the ability to detect deviations, alert operators in real-time, and implement immediate corrective actions. Interoperability with Quality Management Systems (QMS) and Manufacturing Execution Systems (MES) contributes to strengthening overall quality assurance.
Global Context of Biopharmaceutical Production
On a global scale, the biopharmaceutical market represents a growing share of pharmaceutical production, with an estimated annual growth rate of 8 to 10%. More than 60% of drugs currently in development are biopharmaceuticals, and this number continues to rise with the expansion of targeted therapies, immunotherapies, and gene and cell therapies.
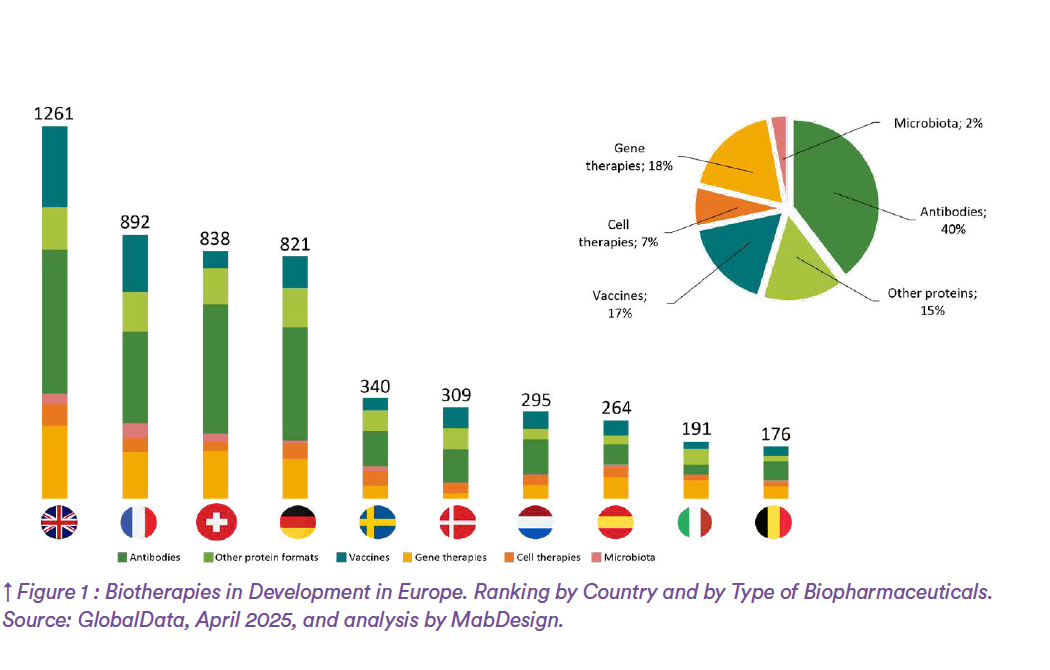
– In Europe, the United Kingdom, France, and Switzerland lead the way in biopharmaceutical development, with a strong focus on therapeutic antibodies and vaccines (Figure 1). Cities such as Basel, Lyon, and Leuven are renowned for their expertise in bioprocessing. France, notably through its “France Biotech 2030” plan, is actively promoting the establishment of innovative national bioproduction sites. Platforms such as Genopole (Evry), Oncopole (Toulouse), and Bio3 (Tours) are clear examples of this rapidly expanding ecosystem.
– In the United States, the concentration of biotech companies, CMOs (Contract Manufacturing Organizations), and clusters such as Boston-Cambridge reinforces their global leadership. The country benefits from a highly dynamic ecosystem combining cutting-edge academic research, private funding, and public-private partnerships. Companies like Genentech, Amgen, and Moderna play a pivotal role in shaping the market.
– In Asia, China, India, and South Korea are investing heavily in state-of the-art facilities, particularly for the production of biosimilars, supported by ambitious national policies and increasing R&D investments. Japan, while more discreet, remains a major player in the field of innovative biotherapies.
Due to their complex nature, biopharmaceuticals require extremely controlled production environments. Unlike traditional chemical drugs, the biological variability inherent to the production of recombinant proteins, antibodies, or cells demands heightened rigor in contaminant prevention and process validation. BFS and SUS technologies address a dual challenge: securing products while making processes more flexible and economically viable.
Additionally, the growing trend of outsourcing production activities to CDMOs (Contract Development and Manufacturing Organizations) adds another layer of complexity. The need for a perfectly managed technology transfer, coupled with maintaining consistent quality requirements between the sponsor and the contractor, makes the adoption of safe, traceable, reproducible, and quickly implementable technologies such as BFS and Single-Use Systems essential. These technologies thus become not only production tools but also strategic levers for global industrial strategy.
Single-Use Systems (SUS): Enhanced Flexibility, Safety, and Efficiency
Single-Use Systems (SUS) have become an industry standard over the past two decades, especially in the field of biologics. They enable the design of flexible, modular, and easily reconfigurable production chains, which is particularly suitable for new formats of personalized and low-volume medicines. A typical single-use system includes disposable cell culture bags, pre-sterilized tubing and connectors, filters, harvest containers, and sometimes even complete disposable bioreactors. All components are assembled in a controlled environment, gamma-irradiated for sterilization, and delivered ready for use.
Using these systems eliminates the need for cleaning and sterilization cycles, known as Clean-in-Place/Sterilization-in-Place (CIP/SIP), reduces the risk of cross-contamination, and significantly shortens batch changeover times. These standardized components serve bioproduction needs efficiently.
The integration of SUS into both upstream and downstream processes is now common. In upstream processes, SUS are used for cell culture, fermentation, and fluid transfer. In downstream processes, they are integrated into clarification, sterile filtration, chromatography, and aseptic filling stages. The adoption of Single-Use Systems is particularly beneficial for companies operating in multiproduct environments, as it enables great flexibility and optimized risk management. It also facilitates rapid process scaling during clinical development phases without requiring heavy investment in fixed equipment.
However, while these components provide enhanced safety and standardization, they also raise sustainability and environmental concerns. One challenge associated with SUS is the management of plastic waste. In response, several manufacturers and industry partners have developed recycling or energy recovery solutions. Compared to traditional processes, the single-use approach may present a more favorable carbon footprint thanks to reduced water, energy, and cleaning chemical consumption.
The adoption of Single-Use Technologies in bioproduction fully aligns with environmental objectives set by European and French regulatory frameworks, such as the National Low-Carbon Strategy (SNBC) and the Greenhouse Gas Emissions Report (BEGES). By significantly reducing water, cleaning chemicals, energy use, and validation times, SUS help limit the overall carbon footprint of biopharmaceutical processes. They also contribute to better flow traceability and simplify production chains, thereby facilitating environmental data collection and compliance with new reporting requirements (scopes 1, 2, and partially 3). Thus, they emerge as effective tools for decarbonization and sustainable optimization in bioproduction.
Contaminants and Impurities: Understanding and Control in Biopharmaceutical Development
As we have seen, SUS and BFS are technologies that help reduce contaminants and impurities. The presence of contaminants and impurities during the development of a biopharmaceutical is a critical risk that must be meticulously controlled. It is crucial to understand their nature, origin, and associated risks.
A contaminant is any unintended foreign substance or agent introduced into the product. There are several types:
– Microbial contaminants such as bacteria, yeasts, molds, mycoplasmas, or viruses;
– Chemical contaminants such as solvent residues, detergents, or lubricants;
– Physical contaminants such as plastic particles, glass shards, or textile fibers.
An impurity is a substance present in the finished product, originating either from raw materials or generated during the process. Impurities can be:
– Product-related (e.g., aggregated forms of a therapeutic protein);
– Process-related (e.g., residual Protein A, host cell DNA).
These contaminants and impurities pose risks to patients. They can induce severe immune reactions (anaphylactic shock, non-specific immune system activation), cause acute or chronic toxicities, or reduce/alter the therapeutic efficacy of the biopharmaceutical.
Risk management therefore relies on a thorough understanding of the processes, rigorous qualification of raw materials, and strict application of Good Manufacturing Practices (GMP).
There are strategies for preventing, detecting, and eliminating contaminants and impurities. Analytical tools have been developed to detect, quantify, and characterize them. These tests, specific to the nature of the contaminant or impurity, are implemented throughout the biopharmaceutical production process up to batch release.
– Chemical impurity detection involves tests such as HPLC, UPLC, and LC-MS/MS.
– Host Cell Proteins (HCP) and residual DNA are monitored through ELISA, Western blot, and quantitative PCR assays.
– Viral contaminants are detected through a panel of methods, including electron microscopy, reverse transcriptase assays, NGS, and antibody production assays.
Continuous monitoring of the production environment is essential to control contaminants and impurities. This includes:
– Airborne particle counting;
– Surface microbiological controls;
– Monitoring of bioburden and endotoxins in intermediate solutions;
– Use of High-Efficiency Particulate Air (HEPA) filtration systems;
– Implementation of validated cleaning procedures;
– Operation in classified areas (ISO 5 to ISO 8).
In Summary
In the face of the increasing complexity of biological processes, mastering contaminants and impurities has become a central strategic issue for the biopharmaceutical industry. Product quality, patient safety, and regulatory compliance directly depend on it. In this context, Blow-Fill-Seal (BFS) and Single-Use Systems (SUS) technologies emerge as major drivers of industrial transformation, offering concrete, agile, and robust solutions to the current and future challenges of the sector.
Their ability to reduce critical contamination points, improve traceability, and limit human interactions in sterile environments represents a major advancement in process security. From a techno-economic standpoint, their adoption leads to a significant reduction in operational and investment costs, particularly by eliminating cleaning steps, enabling flexible batch changes, and speeding up time-to-production. These advantages result in increased productivity and greater industrial responsiveness, which are essential as development cycles become ever shorter.
The growing integration of these technologies into the production of monoclonal antibodies, complex biomedicines, and Advanced Therapy Medicinal Products (ATMPs) illustrates their relevance, both for large-scale manufacturing and for small, personalized batches. Furthermore, their compatibility with current environmental requirements—through carbon footprint reduction and resource optimization—aligns perfectly with the sustainability strategies mandated by new European regulatory frameworks.
Finally, their effectiveness is enhanced when combined with digital tools, Quality Management Systems (QMS), Manufacturing Execution Systems (MES), and advanced analytical platforms. It is within this integrated approach that BFS and SUS technologies fully establish their legitimacy: as technological pillars of a modern, safe, sustainable, and future oriented biomanufacturing industry.
Glossary
BFS Blow Fill Seal
ATMP Advanced Therapy Medicinal Products
BFS Blow-Fill-Seal
CDMO Contract Development and Manufacturing Organization
CIP/SIP Cleaning-In-Place / Sterilization-In-Place
CIP Clean-in-place
CMO Contract Manufacturing Organization
DNA Deoxyribonucleic Acid
ELISA Enzyme-Linked Immunosorbent Assay
GMP Good Manufacturing Practices
HCP Host Cell Proteins
HEPA High Efficiency Particulate Air
HPLC High Performance Liquid Chromatography
ICH International Council for Harmonisation
MES Manufacturing Execution System
NGS Next-Generation Sequencing
PCR Polymerase Chain Reaction
QMS Quality Management System
SIP Sterilization-in-place
SUS Single-Use System
UPLC Ultra Performance Liquid Chromatography
USP United States Pharmacopeia
References
1. ICH Q5A(R1), Q5D, Q6B – Guidelines for the Quality of Biotechnological Products
2. Eudralex Volume 4 – EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use
3. USP <1045> Biologics, <1071> Rapid Microbiological Methods
4. European Pharmacopoeia – Chapters 5.1.6, 5.2.12,6.2.1
5. PDA Technical Reports (TR 26, TR 60, TR 66) on Single-Use Technologies and Contamination Control
6. Scientific publications from PubMed on the prevention of contaminants and impurities in biopharmaceutical processes (e.g., “Risk-based approaches for microbial control”, “Evaluation of viral clearance strategies in downstream processing”)
7. Directive (EU) 2022/2464 of the European Parliament and Council of December 14, 2022, on corporate sustainability reporting (CSRD)
8. European Commission’s “Fit for 55” legislative package
9. National Low-Carbon Strategy (SNBC) – French Ministry of Ecological Transition
10. French Environmental Code, Articles L229-25 to L229-27 regarding GHG emissions reporting
11. Pharmaceutical Industry Decarbonization Roadmap French Ministry for the Economy and Finance
12. “The Importance of Managing the Weight of Pharmaceutical Containers Made by BFS,” La Vague No. 46, June 2015
13. “Industrial Challenges of Widespread Adoption of Single-Use Systems (SUS),” La Vague No. 58, July 2018




