La Vague 53
Low Endotoxin Recovery (LER) is today one of authorities serious concerns regarding pyrogen testing
Lipopolysaccharides / endotoxins are a subtype of pyrogens originating from the cell wall of gram-negative bacteria that are both extremely thermostable and very potent when brought into contact with the human immune system. But they are by no means the only possible pyrogenic contamination – and the growing complexity of biotechnological products increased the risk of having pyrogens present that originate from a variety of microorganisms and other sources.
La Vague 53
Single Use & Stainless Steel: complementarity or fight?
There has been a huge increase in single use apparatus in the biopharmaceutical manufacturing world during the last. Many companies compete in manufacturing production tools. At Boccard where our mantra is “In Stainless Steel We Trust”, we have a different opinion. We believe that plastic and stainless steel are complementary. Let’s take the example of cell culture. Before growing in a huge fermenter (e.g. 1000 L), what is the point of using a stainless steel 20 L fermenter as a first step from an economical point of view? why not use Single Use apparatus in this case?
La Vague 53
Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in BioBLU 0.3c Single-Use Bioreactors
Mesenchymal stem cells (MSCs) are attractive candidates for therapeutic applications, especially in the field of regenerative medicine [1] because – in contrast to embryonic stem cells – they do not pose ethical issues, they can be isolated from various tissue sources, and they reduce the risk of rejection reactions. The doses of human MSCs (hMSCs) needed for clinical trials are estimated at between one and 200 million cells per patient, depending on the disease being tackled [2]. One of the most important challenges in providing hMSCs for curative use is the production of large quantities of cells in a robust manner.
La Vague 53
Qualification approach for the validation of real-word shipping in single-use systems
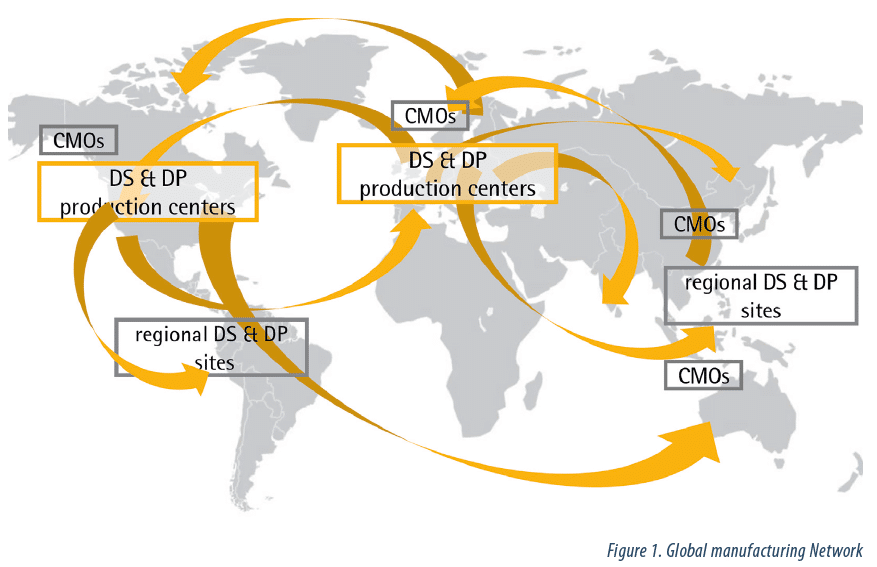
The complexity of biopharmaceutical manufacturing processes requires continuous improvement. As shown in figure 1, the expansion of manufacturing capacity worldwide has resulted in the multiplication of links between production facilities as well as the increasing need for storage or transportation of media, intermediate, BDS, and drug products between sites over the world.
Outsourcing to contract manufacturing organizations (CMOs) offers a solution to the capacity constraints and reduces the total risks associated with building internal capacity; however, a robust and validated manufacturing process (2), including product transportation between facilities, is then required.
La Vague 53
Improving Single Use Bioreactor Design and Process Development. New Research Towards Intensifying Seed- Train & Scale-up Methods Using 5:1 Turn- Down
Immense pressure is being applied to improve process knowledge and execution for those working in the field of bio-therapeutic manufacturing. Bioprocess developers are being tasked to provide more product yield at lower production cost, to decrease the time required to bring new therapies to the patient, and to consistently manage operational risks.
La Vague 53
“Close Collaboration Maximizes Value of Engineered Solutions and Saves Time in Start-Up” The case study of BioMarin Fast-Track project with Verdot Ips²
The case study of BioMarin Fast-Track project with Verdot Ips²
This paper reports a very fruitful collaboration between a Biopharmaceutical company, BioMarin International Ltd and a manufacturer of purification equipment, VERDOT Ips², which contributed to a fast start-up of an installation for manufacturing a recombinant human tripeptidyl peptidase 1 (rhTPP1), for the treatment of neuronal ceroid lipofuscinosis Type 2 (CLN2) disease, also known as Batten Disease.
La Vague 53
Moving One Unit Operation At a Time Toward Continuous Biomanufacturing
Many industries including those associated with generation of power and the manufacture of glass, steel and petrochemicals etc. have demonstrated that continuous manufacturing provides significant benefits and advantages, ranging from reduced capital and operating expenses to greater efficiency and product quality and consistency.
La Vague 53
Frontière Part I/ Part II des BPF : Modalités d’application aux produits biologiques
Suite aux questionnements des Entreprises Françaises de Biotechnologie sur la segmentation proposée par les inspecteurs des parties I et II des BPF vis-à-vis du procédé de production des médicaments biologiques, cet article a pour objectif de proposer une clarification argumentée du cas particulier de ces substances actives biologiques dont la formulation est réalisée lors de la production de substance active et non au moment de la fabrication du produit fini. Ce principe s’applique pleinement aux protéines recombinantes et anticorps monoclonaux, et au cas par cas des procédés de fabrication, pour d’autres types de médicaments de biotechnologie et thérapeutiques innovantes (vecteurs de thérapie génique et vaccins, …).
La Vague 52
Sécurisez le confinement de vos gants.
Il existe deux types de gants pour isolateurs et autres confinements :
• les gants dits “une pièce” qui partent du rond de gant d’épaule jusqu’au bout des doigts
• les gants dits “gantmanchette” qui se composent d’une manchette partant du rond de gant d’épaule jusqu’au rond de gant de poignet puis d’un gant partant de ce rond de gant jusqu’au bout des doigts.
Ce dernier type de montage peut permettre une interchangeabilité du gant en cas de changement de taille ou de perforation accidentelle.