Octobre 2024
La Vague n°83
EU GMP Annex 1 / Maitrise du risque patient ICH Q9(R1) / Digitalisation
Sommaire
- What does 21 CFR Part 11 mean in everyday online analytics?
- Digitalization in cleaning validation an overview of possibilities, challenges, and opportunities for savings?
- EU GMP Annexe 1. Mise en œuvre de la Stratégie de Contrôle de la Contamination
- Impact of the new Annex 1 on Sterile Filling
- The Challenges of Floor Cleaning & Sanitization
- Réduction énergétique des centrales de traitement d’air : comment adapter son monitoring environnemental ?
- USP <922> Water Activity: A Better Approach for Lyo Moisture Determination. Applications enabled by rapid non-destructive headspace moisture analysis of freeze-dried product
- Gestion durable de l'eau dans l'industrie pharmaceutique
- Rapid Testing for Cell & Gene Therapy Products: A Three-Level Approach Using an Automated Solid Phase Cytometry System
The Challenges of Floor Cleaning & Sanitization
Maintaining and improving cleanroom manufacturing operations is required to assure quality production of products. In today’s world, there are many challenges facing cleanrooms and controlled environments and their support areas. Proper cleaning and sanitization of cleanrooms and controlled environments is key to maintaining these facilities at the level they were designed. As an industry, we face challenges to surface cleaning and sanitization.

These include:
1. Manpower shortages to maintain these facilities
2. Need for consistency of cleaning
3. Management of cleaning and operations requiring strict attention to details and techniques
4. Adequate time for cleaning and other ancillary operations
5. Rising cost of operations
6. Safety
7. Changes in regulations
8. Proper use of disinfectants and rinsing.
Each of these challenges can impact the ability to maintain control of a cleanroom. The focus of this article is on proper disinfectant use including understanding of the selection, contact time, rotation and residue with site data focused on the impact of rinsing on environmental monitoring results.
1. Understanding Disinfectant Selection
The most common disinfectants utilized globally in biopharma, pharma, and medical device cleanrooms are quaternary ammonium disinfectants and phenolic disinfectants. Quaternary ammonium disinfectants have been around since the 1800s and are known to be excellent cleaners with deodorizing abilities. Phenolics have been around since the 1800s. As an active ingredient, phenol can have slightly higher levels of kill than quats against some microorganisms such as Mycobacterium.1
The chemicals most widely used in disinfectant solutions attack microorganisms in both broad and specific ways. For example, the extremely low or high pH levels of many of these products may present an inherently hostile environment to certain organisms, while the active ingredients, for example, ortho phenyl phenol or benzalkonium chloride, may act on cell protoplasm or cell surfaces. In other words, not all disinfectants work the same way against all organisms. Further, not all organisms have the same susceptible cell structures. There are real differences in susceptibility between a vegetative bacterium and a spore former. There are also differences between the susceptibility of strains of the same species. Disinfectants should be selected on the basis of performance against common environmental isolates. Further, more than one product must be included in the disinfection program in order to obtain broad-spectrum performance.4 The spectrum should include routine disinfectants, sporicides and alcohols. Detergents may also be considered as a means of removing residue. However, all formulated detergents will leave a residue themselves, so careful consideration should be given to both the detergent chemistry and the selection of a detergent, versus other options that do not leave residues, e.g., alcohol, water.
Routine disinfectants most commonly include those containing phenolic or quaternary ammonium chloride compounds as active ingredients. These products are widely used and well characterized in terms of performance against common bacteria, fungi and viruses. The advantages of using these products are that they are suitable for everyday use on common cleanroom substrates, and they are safe and relatively low in toxicity to the user. Typically, disinfectants are formulated with chelants and surfactants, which render them effective at handling soil loads which would readily inactivate other products, in particular oxidizing chemistries such as bleach and peracetic acid blends. Disinfectants are not, however, effective against spore-forming organisms, such as Bacillus cereus, although some provide performance against challenging molds, such as Aspergillus brasiliensis.
As part of a long-term strategy for control of inherently challenging organisms, such as spore formers and mold, sporicides must be used. These chemistries include bleach, peracetic acid/hydrogen peroxide blends, and chlorine dioxide. Unformulated hydrogen peroxide solutions may also be sporicidal, but this requires high concentrations, 6% or greater and pre-cleaning of surfaces. The advantages of these chemistries are that if applied correctly, they will eliminate all microbial life forms, including the most resistant forms. This makes them well- suited for applications during a catastrophic failure (e.g. power outage) or when bringing a facility back online after a preventative maintenance shutdown, and possibly for more frequent applications as a preventative measure (i.e. weekly or monthly applications). They may also be used more frequently as part of a short-term strategy to address environmental monitoring failures, until such a time as a root cause has been identified and corrective action implemented. However, they should be used carefully as they can be corrosive, even to 316L stainless steel, and challenging for personnel to work with (odor, irritation).
Many disinfectants and sporicides are capable of providing effective reduction against target organisms, as proven by in vitro evaluations (laboratory testing). However, one might observe differences in disinfectant performance between in vitro testing and real-world evaluations. This is because disinfectant performance is affected by a number of factors which may be more challenging to control outside of the test tube. These factors include temperature, contact time, soil loading, substrate, substrate condition (e.g. damage, rouge), presence of biofilm, and application method. Factors such as concentration and water quality are also important – however, these factors are fairly easy to control. Temperature may be more challenging to control depending upon several factors including how long the solution is allowed to be used after preparation. Over time, the solution will work toward equilibration with ambient conditions. Soil loading can be another significant challenge. In some cases, for example media spills, floor drains, etc. pre-cleaning, which can be done with a disinfectant that also has detergent capabilities, should be a precursor to disinfectant application. Perhaps the greatest factor in assuring disinfectant performance is ensuring that adequate contact with the surface has been made during the disinfectant application process. Disinfectants and sporicides used in cold rooms should be evaluated in a coupon study for performance. Cold room temperatures will affect the speed of the reactions of the antimicrobial chemistry in the bacterial and fungal cells. Additional contact time may be required. The dilution rate is also a critical factor that can affect the efficacy of the disinfectant. The best practice would be to use a dilution rate recommended by the disinfectant manufacturer.
Contact time is generally not an issue in CNC, ISO-7, ISO-8 or ISO-6 cleanrooms where disinfectants and sporicides applied with polyester or microfiber mops will obtain 25–35-minute wet contact times. ISO 5 cleanrooms have higher air change rates that impact the wet contact time and may require reapplication of a disinfectant or sporicide to obtain a validated contact time.
Disinfectant rotation has been debated over the past 20 years and is still a topic of conversation. Today rotation can be one robust disinfectant (phenolic or quaternary ammonium) and one sporicide or two disinfectants of similar actives (phenolic or quaternary ammonium) and a sporicide.2,5 Precleaning is typically not required unless there is excessive soiling such as in tissue banking cleanrooms. Periodic rinsing with WFI (Water for Injection) or 70% Isopropanol is recommended based on visual observations of the aesthetics and safety risks (floors becoming sticky, tacky or slippery). There are additional safety risks to be considered with the application of 70% Isopropanol as a rinse – exposure to personnel and fire risk or flash.
The removal of a residue is a challenge today – when to remove the residue, frequency of removal, and how to remove the residue. Many of the disinfectants and sporicidal agents are NOT compatible with each other. This chemical incompatibility can result in a significant visual residue. Removal of this type of residue is difficult and time consuming. When selecting a disinfectant and sporicide, the substrate and disinfecting agent compatibility must be considered.
2. Cleaning Events
Over the past two years, there have been major concerns with floor cleaning. These issues reported have far surpassed the level of consequence in past cleanroom history. During this time period, over 150 events in North America have been documented by the authors involving the common factors:
a. Heavy residual
b. Visible flaking of residual
c. Severe damage to floors
d. Safety
Initially, the problems appeared to be random, some site specific, some floor specific, and others – total unknown. As the cases became more prevalent, the concern increased, and the driving question became – What Changed? Why Now?
The Institute of Environmental Sciences and Technology published a document, Recommended Practice 18: Cleanroom Cleaning and Sanitization which includes the techniques and methods on mopping and wiping all cleanroom surfaces, furniture and equipment. These methods have been tested worldwide by round robin in the committee and used in all classes of cleanrooms over the last four decades. The authors began a full investigational analysis on the specifics of each event.
3. Analysis
In reviewing and analyzing the 150 sanitization events, the following list of factors were reviewed:
a. Type of flooring
b. Frequency of cleaning and sanitization
c. Tools
d. Techniques
e. Disinfectant
vi. Type
vii. Contact Tim
viii. Concentration
ix. Residue removal
f. Environmental conditions – temperature and humidity
4. Case Study Background
The cleaning events prompted the authors to find users that would perform “testing” under a protocol condition to determine why these issues were occurring and the impact of these events on the cleanroom marketplace. After months of efforts, twenty-five commercial manufacturing companies (25) in five different sectors of the cleanroom marketplace agreed to perform testing to determine the impact on environmental monitoring data of residual removal. The companies performed the sanitization utilizing their staff (contract or internal), their choice of supplies and their validated disinfectants. The following criteria are the background for this testing.
a. Floors
Flooring surface condition especially of cleanroom flooring and the sealants used on the flooring can create issues. In researching the 150 events, the writers observed that some cleanroom flooring needs to be replaced because it is damaged or the sealant on the floor is breached. There have been incidents in which the sealant has been breached due to the pooling of disinfectant. Breached surfaces can have an adverse impact on cleaning and sanitization efficiency and potential environmental monitoring risks.
With the concern for comparable data, the authors examined the floors of the 25 test case sites prior to the case study testing. The floor types in these case studies were 97% aggregate concrete with type 2 and 3 finishes. One floor had a type 4-finish, and another floor was a welded sheet vinyl.
b. Frequency of cleaning and sanitization
The GMPs define the cleaning and disinfection requirements for cleanrooms based on product quality, EM data trending, and
cleanroom classifications. There is no defined“regulatory” requirement for frequency – just limits for EM and interpretation of adherence to GMP. Under the ISO standards, there is no frequency requirement
– only risk and impact assessment. The ISO 14644-2 document for particulate monitoring frequency also refers to risk, unless there is a regulatory requirement. The microbial monitoring frequency is regulated based on classification and processing types, but the precise cleaning frequency is not regulated. The “c” in cGMP for frequency is a variable for the classes of cleanrooms
– depending on the product requirements, open or closed, rotation of products within the same room, population density, cleanroom design, EM data, etc.
The frequency of cleaning varies from daily to once per shift. The members that participated in the case studies all performed the identical frequency of cleaning and sanitization.
c. Tools
All mops were sterile and met cleanroom requirements for acceptable materials, i.e. microfiber. The mop buckets were either sterilized or sterile liners were used. All mop handles were sterilized.
d. Techniques
All twenty-five case study participants followed one of the two techniques detailed in IEST Recommended Practice 18: Cleanroom Cleaning and Sanitization. The efficiency of these methods has been published and are referenced in the document. The two methods are “pull-lift” and modified figure “8”.6
e. Disinfectants
All disinfectants were validated for efficacy. The contact times were appropriate to each case study participant per their validation. Dilution of the disinfectant met the validated concentration for each case study participant. All disinfectants were sterile.
How clean a surface appears is extremely subjective and is based on the background, visual acuity and understanding of contamination control. Residue follows the same type of critique. What appears as an acceptable residue to one person, may not be acceptable to another. Disinfectants leave a residue on the surface. The major regulatory concern is a build-up of this residue that could lead to flaking and particle concerns in the cleanroom and potentially on a product. Our major concern in today’s cleanroom is “why” the build-up of residue has changed!
Over the decades, residue has not been a major issue when firms have followed the proper cleaning techniques and utilized proper tools. With the impact of COVID-19, companies have struggled in securing trained personnel. Cleaning has become a rapid uncontrolled process that leads to disinfectant pooling. The impact of poor techniques, inadequate tools, and improperly trained personnel has resulted in additional residue.
The case studies will demonstrate the impact of residue and the removal on surfaces.
f. Environmental conditions
The temperature and humidity conditions were within similar ranges for all the cases 65-68° F and 40-48% relative humidity.
5. Case Study / Test Protocol #1
The purpose of this testing was to determine the results on a floor after operations without the removal of the disinfectant residual (no rinse). The test location was an ISO 8 airlock, non-clean side. Floors were sampled using RODACRODAC plates. All plates were incubated in accordance with United States Pharmacopoeia USP General Information Chapter <1072> Disinfectants and Antiseptics.2
The airlock was “in-operation” after cleaning and sanitization. The normal transfer activities were performed per the user’s SOP using validated disinfectants and proper cleanroom techniques.
Floors were mopped using sterile mop heads, approved validated solutions – quat or phenol, and techniques per IEST RP-18.6 Contact time was based on the validation of that location.
The following were the steps of the test protocol:
Step One: floors were mopped with approved sanitizer solution at the validated dilution rate and held for the contact time.
Step Two: floors were monitored after contact time for at-rest baseline data.
Step Three: operations were performed for that shift.
Step Four: at the shift end, floor testing was performed for the set location as defined by the test protocol (see Figure 3). All testing was performed using the same dimensions of an airlock. The size area tested was five feet in width and ten feet in length. Any participants with larger airlocks only tested in the center area and the side areas were not sampled. This allowed for the same area to be tested in all case study participants.
Step Five: after sampling, floors were re-mopped with approved sanitization solution.
Five different types of operations and five manufacturing sites were represented. All airlocks were used for the transfer of materials into the cleanrooms. None of the airlocks in this study were used as exit for employees or materials. The companies represented were:
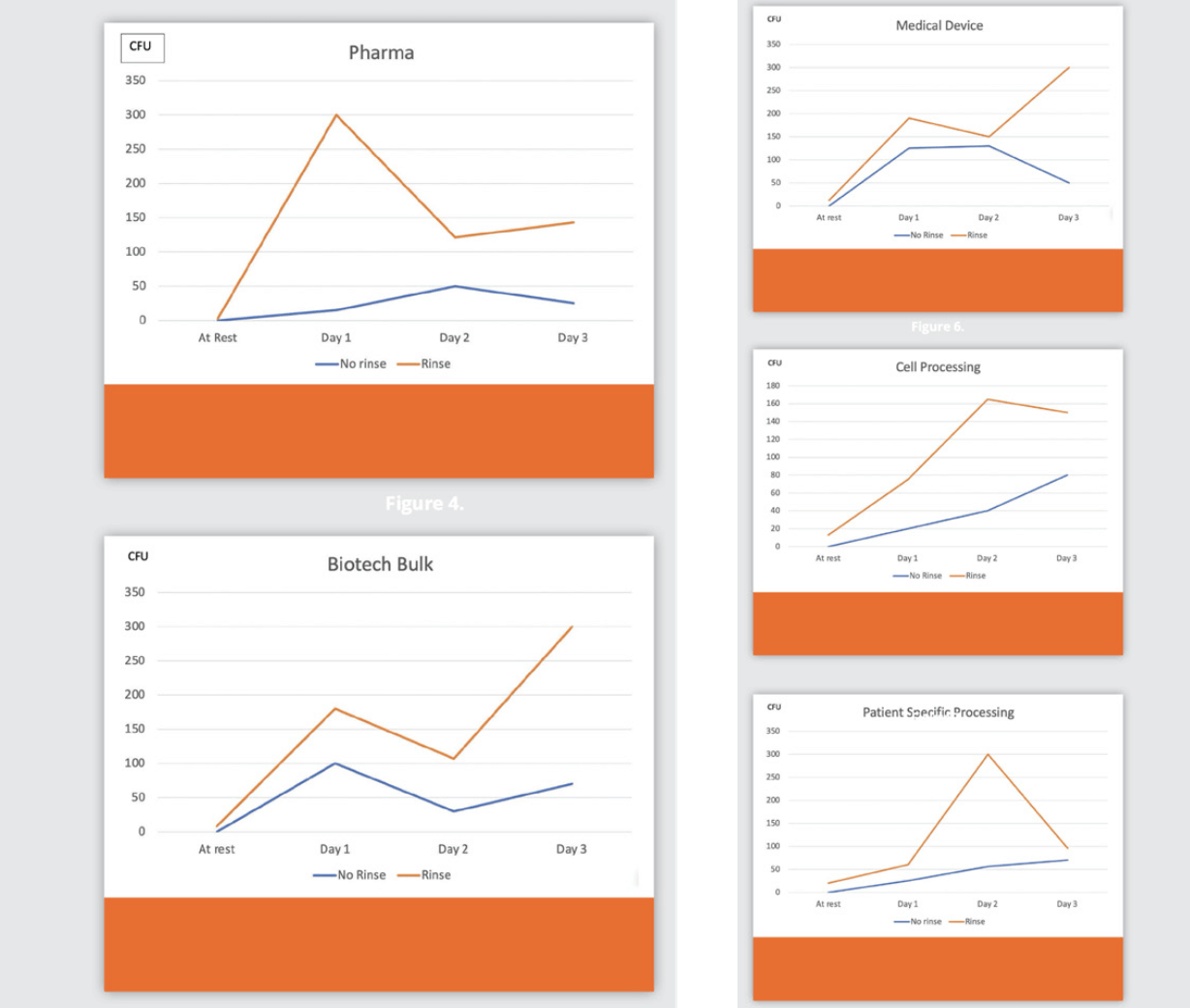
Client A Pharmaceutical ; Client B Biotech (bulk); Client C Medical Devices ; Client D Cell processing ; Client E Patient specific processing
Data
The data (Figures 4-8) is a summary of all plates by type of operation. It is important to note that no company exceeded action level limits for the floor samples, no particle counter action levels were noted, and no pressure alarms were breached or exceeded.
The numbers are variable due to several factors:
1. Number of transfers in the airlock
2. Types and sizes of transfers
3. Number of personnel required to place an item on the non- clean side of the air lock. As an example, in one incident, two people were required to move a tank into the airlock.
The at-rest represents the results after sanitization allowing for validated contact times. All floors were in a dry state for the at-rest testing. The testing occurred prior to any operational personnel entering the airlock. The monitoring technician wore sterile boots over their shoe covers and used sterile gloves for the sampling. The colony forming units (CFUs) are per plate.
The testing results shown for the end of shift were performed at least eight hours after the at-rest testing. The same monitoring technician sampled the area wearing new sterile boots over their shoe covers and sterile gloves for the sampling.
6. Case Study / Test Protocol #2
The purpose of this testing was to determine the contamination levels on a floor after operations with the removal of the disinfectant residual by rinsing after the contact time.
The test location was the same ISO 8 airlock, non-clean side. Floors were sampled using RODAC Plates and were incubated in accordance with the same procedure as Test Protocol #1.
As with Test Protocol #1, the airlock was “in-operation” after cleaning and sanitization. The normal transfer activities were performed per SOP using validated disinfectants and proper cleanroom techniques. Floors were mopped using the identical tools, techniques, solutions and contact times as reported in Case Study #1.
The following were the steps of the test protocol:
Step One: floors were mopped with approved sanitizer solution at the validated dilution rate and held for the contact time. Floors were rinsed with WFI using new sterile tools and techniques as identical to the mop step and allowed to dry.
Step Two: floors were monitored after contact time for at-rest baseline data.
Step Three: operations were performed for that shift.
Step Four: at the shift end, floor testing was performed for the set location as defined by the test protocol (see Figure 3). All testing was performed using the same dimensions of an airlock. The size area tested was five feet in width and ten feet in length. Any participants with larger airlocks only tested in the center area and the side areas were not sampled. This allowed for the same area to be tested in all case study participants.
Step Five: after sampling, re-mop floors with approved sanitization solution.
The results are shown in Figures 4-8. The same industries participated in this case study.
The at-rest represents the results after sanitization allowing for validated contact times, followed by a WFI rinse. No floors were tested with any visual wetness. The testing occurred prior to anyone entering the airlock. The monitoring technician wore sterile boots over their shoe covers and used sterile gloves for the sampling.
The testing results shown for the end of shift were performed at least eight hours after the at-rest testing. The same monitoring technician sampled the area wearing new sterile boots over their shoe covers and sterile gloves for the sampling.
The data is a summary of all plates for each type of facility. It is important to note that several companies exceeded action level limits for the floor samples. However, no particle counter action levels were noted, and no pressure alarms were breached or exceeded.
The numbers are variable due to several factors:
1. Number of transfers in the airlock
2. Types and sizes of transfers
3. Number of personnel required to place an item on the non- clean side of the air lock. As an example, in two incidents for “B” type customers on day 3, 2 people were required to move a tank into the airlock.
7. Data Comparison
Figure 4 through Figure 8 show the results by industry without a rinse and with a rinse prior to operations. The TNTC is displayed on the graphs as 300 cfus.
8. Final Summary
Several observations can be made from these studies:
1. The difference between the rinse and no rinse after sanitization and prior to reapplication of the disinfectant is very evident – the disinfectant effectiveness was no longer adequate to control contamination when the rinse removes the disinfectant after contact time. The case study did not address the frequency of residue build up removal that may be needed; this frequency may need determined based on a risk assessment, and increased if there is a risk of particulate contamination.
2. Residue is of significant concern if the application does not follow the proper techniques of application.
3. Training, and proper tools are required to control contamination. Sanitization procedures must be supervised and audited to ensure competency.
4. Airlocks are difficult to manage and the amount of contamination risk is dependent on many factors. In these 25 cases, the protocols were correct, the techniques were appropriate, but the number of transfers could exceed the airlock’s ability to maintain the levels of control required. Users must review the sanitization frequency of these areas to determine if additional floor sanitization is needed.
Partager l’article

Anne Marie DIXON-HEATHMAN

Références
1. McDonnell, G. and Hansen, J. (2020) Block’s Disinfection, Sterilization, and Preservation. 6th ed. Wolters Kluwer Health.
2. USP 43 <1072> Disinfectants and Antiseptics.
3. Sterile Drug Products Produced by Aseptic Processing-Current Good Manufacturing Practice 2022.
4. Polarine, J., Sartain, E., and Bartnett, C., (2015) Cleaning and Disinfecting Cleanrooms, Environmental Monitoring Vol. 7. PDA/DHI Publishing.
5. PDA Technical Report #70, Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities, 2005.
6. Institute of Environmental Sciences and Technology, IEST RP-CC018.5, Cleanroom Cleaning and Sanitization: Operating and Monitoring Procedures
7. Dixon, Anne Marie, (2009), Cleaning and Cleaning Validation, chapter 11, Cleaning of Non-Product Contact Surfaces. PDA/DHI Publishing
8. Dixon, Anne Marie (2017), Aseptic and Sterile Processing, chapter 13, Cleaning and Sanitization for Aseptic Processing. PDA/DHI Publishing
9. ISO 14644-5, Cleanrooms and Associated Controlled Environments: Operations.