Sommaire
- La restriction des PFAS dans l’industrie : enjeux réglementaires et impacts sur l’industrie pharmaceutique
- La technologie Blow-Fill-Seal dans l’industrie pharmaceutique : performance, applications et durabilité
- Key Allies in Preventing Contaminants and Impurities in Bioproduction
- Choosing the right vial: packaging sterile drug products with foresight
- Combination Products in the United States and European Union: Differences and proposed strategy to prepare common CTD Quality Module 3
- Blood plasma processing. When every drop counts
- L’analyse de la normalité en Continued Process Verification
- Qualification of impurities
- Pharma 2052
Combination Products in the United States and European Union: Differences and proposed strategy to prepare common CTD Quality Module 3
Recent years have been marked by a high increase in the number of marketed injectable products due to the strong development of biological and biotechnological products.
This led to an important expansion of combination products to facilitate administration of the products
to patients. Guidance’s to help build the marketing authorization dossiers both in the United States (US) and the European Union (EU) (our regions of interests for this article) exist since some years now and are globally harmonized in terms of the expected content of information.

However, differences exist in the terminology used as well as in terms of format and location of the information in the dossier due to different marketing authorization pathway.
Only integral products according to the European Medicines Agency (EMA) defi- nition or a combination product with a primary mode of action induce by a drug or a biologic according to the Food and Drug Administration (FDA) will be in the scope of this article.
Regarding the regulatory factors involved for both regions, the main guidelines as well as the terminology will be further detailed.
The main differences between the American and European approaches will be displayed and a strategy to circumvent these differences will be proposed.
1. What is the landscape for Combination Products in the US and EU?
a. Who regulates Combination Products?
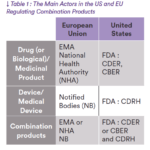
This is probably one of the main diffe- rences between the US and EU. While in the US a combination product is fully regulated by FDA (with the support of different centers), in Europe two different and independent entities are involved. The main actors for both regions are mentioned in Table 1.
b. What are the main regulatory texts to consider?
In the US, two main guidelines can be mentioned:
– eCTD Technical Conformance Guide,
– Current Good Manufacturing Practice Requirements for Combination Products.
In addition to these guidelines, the 21CFR Part 3, 4, 211, and 820 should be considered.
In the EU, there is mainly one guideline (Guideline on quality documentation for medicinal products when used with a medical device) that could be seen as an equivalent of the FDA’s eCTD Technical Conformance Guide.
Additionally, the Medical Device Regulation, the Directive 2001/83/EC as well as the Questions & Answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect to the implementation of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/745 and (EU) 2017/746) should also be mentioned.
c. How is a Combination Product defined?
The definition proposed by FDA in the cGMP Requirements for Combination Products Guideline is based on 21CFR Part 3 and is defined as follows: “a product composed of two or more different types of medical products (i.e. a combination of a drug, device, and/or a biological product with one another)”.
Whereas the EMA defines in the guideline on combination products a “Drug-Device Combination Product (DDC) as a medicinal product with inte- gral and/or non-integral medical device/ device component(s) necessary for admi- nistration, correct dosing or use of the medicinal product”.
From the definitions of a combination product proposed by the FDA and the EMA, it seems that the definition use by FDA is broader than the one presented by EMA.
2. What are the main differences between the US and EU for a Combination Product?
As mentioned in the previous section, one of the main differences between the US and EU is the submission pathway (see Table 1).
While in the US everything lies with the FDA, and one submission is needed, in the EU, two completely separated entities (a Notified Body and the EMA or a National Health Authority) review diffe- rent parts of the dossier which leads to a submission in two steps.
In addition to the differences in terms of procedures, differences in the terminology used can also be noted.
Indeed, in the FDA guidelines, the terminology used includes all types of combination products whereas in the EMA guideline defines different types of combination products but always with a primary mode of action as a drug.
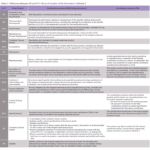
Another difference that can be highlighted is the recommendation made in terms of location of the information in Module 3 which is summarized in Table 2.
Given the differences in terms recom- mendation by the FDA and the EMA for the location of the information, it seems that two different Module 3 will need to be prepared: one for each region.
This means more time and resources need to be allocated not only for the preparation of the initial submission but also for post-marketing activities, as two dossiers will need to be managed.
Is there not a way to harmonize and streamline the submission preparation when both the US and EU are involved?
3. Can a common Quality Module 3 be created for both regions?
As explained previously, the European and American approach are not completely harmonized regarding the pathway to review and present the data within Module 3.
However, and most importantly, the FDA and the EMA are aligned in terms of the expected content (i.e. the studies to be performed and the data to be generated).
Therefore, it is believed that one Module 3 can be prepared and submitted to both the US and EU.
It is suggested to use the use the FDA eCTD Technical Conformance Guide guideline as reference for the location of the information, and as recommended by this guideline, to include most of the information linked to the device Section 3.2.R by creating a specific 3.2.R Device section.
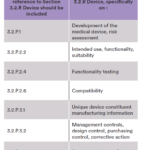
Furthermore, to help the reviewer during the assessment of the dossier, it is suggested to make cross-references to Section 3.2.R Device in all Module 3 sections where it is normally expected to have information linked to the device as summarized in Table 3.
In addition, it is recommended to prepare a reviewer’s guide, as recommended by FDA, to be submitted in Module 1, that will also help the reviewer locate quickly the most important information.
By adopting this strategy of one dossier, based on the FDA guideline, the only adaptation that will need to be performed for the EU is the addition of the Notified Body Opinion (as a standalone document) due to this specific review process.
4. Conclusion
As explained through this article the landscape for regulation of combination product differs between the US and EU but the expected information being the same these differences can be overcome.
It is therefore possible to have a harmonized dossier that can be submitted in both regions by including the requested information in Section 3.2.R of the Module 3.
When using this strategy, it is important to add the proper cross-references to Section 3.2.R in the appropriate Module 3 sections as well as in the reviewer’s guide.
Having most of the information in Section 3.2.R avoids reworking the dossier for an EU or US submission, allows more flexibility for submissions outside of these regions where the product will not necessarily be regulated as a combination product, and facilitates post-marketing activities by limiting the number of sections that need to be updated.
References
– FDA Current Good Manufacturing Practice Requirements for Combination Products
– FDA eCTD Technical Conformance Guide. Technical Specifications Document
– 21CFR Part 3, 4, 211, and 820
– EMA Guideline on quality documentation for medicinal products when used with a medical device (EMA/CHMP/QWP/BWP/259165/2019)
– Questions & Answers for applicants, marketing authorisation holders of medicinal products and notified bodies with respect to the implementation
of the Medical Devices and In Vitro Diagnostic Medical Devices Regulations ((EU) 2017/745 and (EU) 2017/ 746)
– EU Medical Device Regulation 2017/745 – EU Directive 2001/83/EC





