Summary
- Outsourcing bioproduction of biomedicines in France
- Outsourcing supplier audits: The keys to success
- Selecting container closure components with confidence: A data-driven approach to CCI
- The Art of Understanding Language: The Evolution of Natural Language Processing
- Microbial Monitoring RABS Gloves: Unravelling the Implications of Directional Use
- General Considerations on Bacterial Endotoxins & USP Approach to Developing GC <86> Bacterial Endotoxins Test Using Recombinant Reagents
- Bacterial Spore Formers in Disinfectant Efficacy Testing
- Avoiding product oxidation by H2O2 in isolators. It all depends on the right analyses!
Avoiding product oxidation by H2O2 in isolators. It all depends on the right analyses!
Biopharmaceuticals have become an integral part of many therapeutic areas, and their application possibilities continue to increase. Accordingly, the need for protective measures for these parenteral products is also rising. Isolators are highly popular in aseptic manufacturing. But they also entail risks, for example through the effects of hydrogen peroxide (H2O2) which is used for decontamination. Thomas Kosian from Syntegon and Felix Heise from Merck (EMD Serono) explain how these risks can be counteracted with profound and targeted analyses.
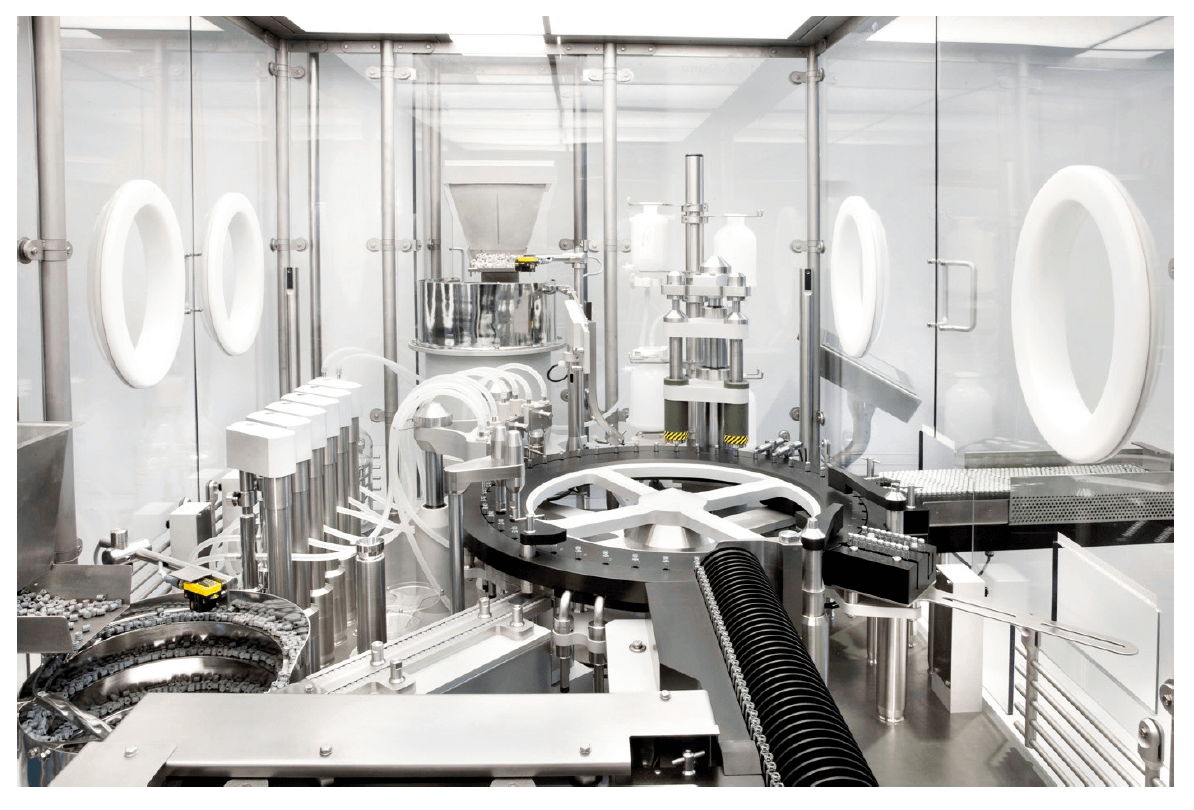
Thanks to scientific and technical advances, groundbreaking developments are taking place in biotechnologically produced drugs, for example for the treatment of cancer, autoimmune diseases, or rare diseases affecting only a small patient population. However, these highly efficacious and sometimes very toxic drugs require special safety precautions and hermetically sealed production processes to protect humans and pharmaceuticals from each other. At the same time, especially newly developed drugs which are often produced in smaller batches require a high degree of flexibility and modularity. Integrated air management and optimized bio-decontamination play a fundamental role in the flexible integration of isolators into existing building and clean room concepts, while ensuring safe production processes.
1. H2O2 Safety and risk factor at the same time
Gassing with hydrogen peroxide (H2O2) has become a standard for the automated biodecontamination of isolators. H2O2 is a rather stable liquid compound of hydrogen and oxygen – a strong oxidizing agent which is particularly suitable for decontamination due to its broadspectrum effect. After the decontamination cycle, the remaining H2O2 is either decomposed by catalysts or flushed from the isolator by intense ventilation with fresh air to achieve an acceptable residual concentration. The rapid growth in the biotech sector has increased the requirements on filling machines and isolators, as many products are quite sensitive to residual H2O2. The aim is to attain a level inside the isolator typically below 0.5 ppm (parts per million) before filling can begin. However, the exact limit depends largely on the sensitivity of the products and can be much lower – even down to approx. 0.03 ppm.
Despite an extensive aeration phase, a part of the H2O2 remains in the isolator atmosphere and may even condense on surfaces such as isolator internal side or filling equipment. Once it gets into the liquid pharmaceutical, it might lead to oxidation. While a residual concentration of 0.5 ppm can be achieved in a standard isolator with an aeration time of about one hour, it can take several hours to reduce the residual concentration to 0.03 ppm for especially sensitive biopharmaceutical products. This leads to filling line downtimes which should be kept as short as possible, particularly in small batch applications with frequent product changes and/or manufacturing in campaign mode.
2. Biotherapeutics as a practical example
Biological molecules like hormones or antibodies are easily oxidizable. A modification of sensitive amino acid residues like methionine, tryptophan, and cysteine affects their physico-chemical properties and possibly also the secondary and tertiary structure of the protein with a potential impact on the product’s efficacy and/or safety. The sensitivity of a drug product depends on many factors, such as the individual properties of the active ingredient, e.g. the type, number,and location of oxidizable amino acid residues and their specific impact on pharmacodynamics and/or pharmacokinetics. Formulationrelated parameters like the concentration of the active ingredient and the presence of oxidation-sensitive or antioxidative excipients such as polysorbates and L-methionine, respectively, also have an impact.
Moreover, the diameter of the container and particularly the (effective) size of its opening influence the diffusion of H2O2 into the product solution.
Besides these product-related factors, filling equipment, technology, and the process itself also play an important role. For example, close attention must be paid to the exposure time of open and partially stoppered products to residual H2O2 during filling, machine stops, or buffering of filled units inside the isolator before loading into the freeze dryer. Silicone tubing is known to absorb and slowly release H2O2 an might lead to relevant migration of H2O2 into the product solution, for instance during line stoppages. During filling, nitrogen flushing and overlay may help to reduce H2O2 residuals inside the container.
3. Analyses are crucial
What needs to be considered when decontaminating with H2O2?
At first glance it seems reasonable to define a universal target according to the most sensitive product. Depending on the specific exposure situation, 0.03 ppm can already affect certain molecules. If there is no experience with this kind of products and risks, pharmaceutical manufacturers may want to remain on the safe side with this cautious approach – and usually end up far below the required concentration level. By implication, however, this leads to a longer than necessary aeration phase, which costs time and limits the system’s availability.
The better and by far more efficient solution is to familiarize oneself with the most important parameters. How does the product react to H2O2?
Which residual concentration is permissible while avoiding an oxidation risk? Unfortunately, only the “airborne” concentration can be determined during the ongoing process by means of online measuring systems, which continuously monitor the decontamination, aeration/ venting, and production phases. In addition, sensors for routine monitoring typically have a limited sensitivity of 0.1 ppm, which is not sufficient for very susceptible products. Here the decontamination and aeration cycle must be validated using special and very sensitive sensors which are typically not present on manufacturing equipment.
4. Many factors determine the residual concentration
On the other hand, the concentration of H2O2 in the product solution can only be determined by offline experiments and is difficult to track in ongoing production. Nevertheless, studies can establish a relation between concentration in the air and in the solution. Using a fixed airborne H2O2 concentration and a variable exposure duration, it is possible to determine the uptake into the product or a surrogate and to simulate the conditions on the machine. This enables pharmaceutical manufacturers and equipment providers to fine-tune the decontamination process of existing production lines. In the case of new lines, extensive product knowledge helps to adapt the isolator even more precisely to the specific requirements.
When designing or optimizing an isolator, it is important to know all relevant product, process, and equipment parameters. Once the acceptable H2O2 concentration has been determined for a specific product and process, it may not be exceeded in the qualified and validated decontamination process. Again, numerous factors play a role – from the type of container and its filling volume, the temperature inside the isolator, changes in air volume and H2O2 concentration over time, to the process duration and the exposure time of the stoppers and containers. Materials used for filling machines and isolators also offer optimization potential. For example, certain materials such as silicone tubes or seals are known to absorb H2O2. Since they only release it again very slowly, their use should be reduced to a minimum if very sensitive products are to be processed.
5. Detailed studies
The ideal situation, which is not available to every development laboratory, is the simulation of exposure to “airborne” H2O2 in a test isolator. Open products can be exposed to defined concentrations of H2O2 for different durations.
Moreover, process parameters like time between filling and stoppering, line stoppages due to interventions, and buffering of partially stoppered containers when loading the freeze-dryer can be considered. However, due to handling issues (mainly manual sample preparation), such a study often does not generate enough samples for a subsequent stability study. Hence, splitting the assessment into an uptake and a separate spiking study is the more feasible alternative.
In this case the uptake study serves to determine the quantity of H2O2 absorbed by a surrogate liquid (typically water) under the same exposure conditions and in the same configuration (fill volume, container, partial stoppering) as the product in the test isolator. Only the concentration of dissolved H2O2 is quantified using a sensitive analytical assay, for example a peroxidase assay with a fluorogenic substrate. The data is then used to spike product solutions for the stability study with diluted H2O2 yielding the same final concentrations as measured in the surrogates. Since H2O2 can be consumed by the product rather quickly, a water control which is treated the same way as the product samples allows to check if the spiking procedure has been successful.
This split approach also allows pharmaceutical manufacturers to outsource uptake studies to their equipment supplier, provided the latter has the required process and analytical technology and expertise. In this case only the spiking and stability studies are performed in-house – an approach that Merck and Syntegon have successfully used for different projects.
6.Experience makes for the ideal process
Despite the high sensitivity of certain biopharmaceuticals, hydrogen peroxide remains the method of choice for decontaminating isolators. Based on experience and the appropriate studies, it is possible to determine very precisely how a particular filling line must be designed and operated to decontaminate it both safely and efficiently. Especially with new lines, decontamination, product oxidation, and the permissible residual concentration of H2O2 should be considered during the design and engineering phase to minimize its effects on cycle times.
The more biopharmaceuticals are brought to market, the more extensive the experience in dealing with these molecules and their challenges in the fill-finish process will become. Hence, working with a reliable partner with many years of experience in process and measurement technology, isolator design, and product testing helps to successfully master these challenges.