Summary
- Why and how to carry out thermal characterization of a product before lyophilization?
- How should a risk analysis be conducted for contamination in a lyophilization process?
- CIP evolution in freeze dryers. Optimize turnaround time & water consumption
- Silicon oil detection in pharmaceutical freeze dryer by mass spectrometry
- Mass spectrometers. Experience feedback
- En 17272. Everything you should know about the new standard on airborne room disinfection by automated process
En 17272. Everything you should know about the new standard on airborne room disinfection by automated process.
The new European standard EN 17272 is the reference document for tests to be used by suppliers of automated airborne surface disinfection systems to claim defined antimicrobial activity. The standard is based on the French standard NFT 72-281, but contains additional test requirements such as uniformity of biocide distribution, total airborne disinfection contact time, the specific microorganisms used and the test volumes of the enclosure.

This document deals with the context of the development of the standard and provides instructions on its application.
An automated airborne surface disinfection system diffuses a product in the gaseous state, by steam or compressed air from a device used with no humans present.
The most commonly used technologies are surface disinfection systems that vaporize or nebulize hydrogen peroxide and formaldehyde fumigation devices. However, the standard applies also to less well known technologies such as total release foggers (containers of product placed in an enclosure and activated to release the product), as well as ozone surface disinfection systems.
In France, airborne surface disinfection technologies have been subject to standards for many years. The standard NFT 72-281 was initially developed and introduced in the 1980s and was then updated several times, up to its last revision in 2014.
In 2010, the European Committee for Standardization (CEN) judged it useful to create a European standard to evaluate the efficacy claims of the many automated airborne disinfection systems available on the market. The systems in question aim to disinfect surfaces in pharmaceutical facilities and the sensitive areas of hospitals. Drawing on the existing French standard NFT 72-281 accepted by the industry, an expert group led by France developed a new standard – EN 17272 – over several years. The standard was the work of a working group which sets out requirements in support of claims in the medical, veterinary, industrial and agrifood fields. Although heavily based on NFT 72-281:2014, the new standard aims to respond to certain limits identified in the NFT 72-281 standard and to clarify certain criteria, such as the test volume of the enclosure.
What are the four main differences between the EN 17272 and NFT 72-281 standards
1. Distribution test
The NFT 72-281:2014 standard requires that the biological indicator is placed at a defined distance from the automated airborne disinfection system relative to the volume of the enclosure in which the tests are carried out. The indicators are positioned vertically, at a height of between 1.0m and 1.5m above the floor, facing the device. Although this configuration indicates that the disinfectant and its distribution technology are capable of achieving a certain level of microbiological reduction in front of the device at a height of 1.0 to 1.5 m, it does not provide confirmation that the same level of microbiological reduction is achieved behind the device, or in the corners of the enclosure, or on surfaces which may be horizontal, particularly those that face the floor. Most automated airborne disinfection systems claim a decontamination action on all exposed surfaces or on the room as a whole. The criterion of the NFT 72-281 standard did not allow evaluation of such a claim. A distribution test was included in the EN 17272 standard in order to evaluate the uniformity of automated airborne disinfection at all points on all surfaces of the enclosure and under conditions where the effects of gravity or reduced distribution may affect the process. In total, 8 biological indicators each containing > 1 x 106 CFU of Staphylococcus aureus are positioned in the corners of the enclosure. Two indicators are located in a lower corner of the enclosure – one is oriented towards the wall and the other towards the ceiling.
Two other indicators are located in the upper corner of the enclosure, diagonally opposite the first two, one facing the ceiling and the other the wall. Two indicators are located in the upper corner of the enclosure, facing those located in the upper corners. One indicator faces the floor and the other faces the wall. The last two indicators are located in the lower corner of the enclosure, diagonally opposite those located in the other lower corner – once again, one facing the floor and the other facing the wall. The arrangement of the indicators is represented in the graphic in Figure 1. Distance from corners = 50 cm (15 cm for small enclosures).
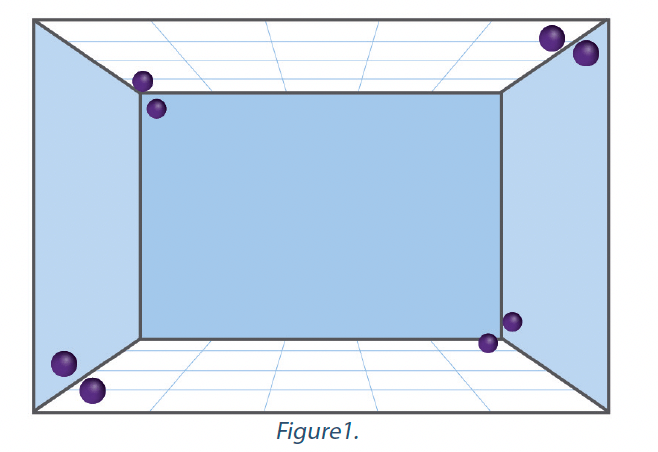
The automated airborne disinfection system must achieve a reduction of more than 5-log on each of the eight biological indicators. If the logarithmic reduction required is not achieved on all indicators, the system cannot claim to execute the standard. The cycle used to carry out the distribution test must be identical to the cycle used in the basic efficacy test and the application device must remain in the same place in the test enclosure.
2. Microorganisms
The test microorganisms in the EN 17272 standard are substantially similar to those in NFT 72-281 and to the EN disinfectant efficacy standards. The specific microorganisms used in the test depend on the claims of the manufacturer relating to use of the technology. To claim compliance with the standard, a manufacturer must, as a minimum, satisfy the requirements relating to bactericidal, yeasticidal tests, and distribution. If a manufacturer wishes to support a claim, for example, of activity against viruses in a hospital setting, it must satisfy the bactericidal, yeasticidal and virucidal requirements for the medical field in addition to the distribution test. Table 2 presents the test microorganisms and the logarithmic reductions required in support of a claim of compliance.
Specific test microorganisms are applied for different areas of use and although logarithmic reduction requirements are generally the same for all areas of use, a claim of sporicidal activity necessitates a logarithmic reduction of 4 in the medical field. Efficacy against the Murine Norovirus and the Adenovirus Type 5 is necessary to support a claim of virucidal activity in the medical and industrial fields, while the small non-enveloped Porcine parvovirus is necessary to support a virucidal claim in the veterinary field. Efficacy against Acinetobacter baumanii must be established in the context of tests to support a claim of bactericidal activity in the medical field.
3.Enclosure volume
The NFT 72-281:2014 standard was not precise regarding the volume of the enclosures in which the tests had to be performed. The standard indicated that a volume of 30 m3 to 150 m3 had to be used to establish a claim of efficacy but it also provided information on the testing of small enclosures of up to 10 m3. The test requirements relative to small enclosures were not clear and 10 m3 was not representative of small enclosures such as microbiological safety cabinets, isolators, transfer airlocks, etc. The standard also required the manufacturer to carry out tests in a large enclosure of more than 150m3. This requirement was impractical, unrealistic and did not supply useful information. Carrying out a test according to NFT 72-281 in a large empty enclosure does not assess the efficacy of decontamination in the enclosure but assesses solely the location of biological indicators in front of the generator. The results obtained are applicable to the enclosure tested under environmental conditions at the start of the test. They may not be transferable to another enclosure of equal volume, but with a different internal configuration, different construction materials and surface-volume ratio. There is little or no value in the test.
The EN 17272 standard improves the clarity of test requirements relative to enclosure volume. Concerning small volume enclosures, manufacturers must carry out tests in an enclosure with a volume of between 0.25 m3 and 4.0 m3. For claims of use in enclosures larger than 4 m3, manufacturers must use a room with a volume of between 30 m3 and 150 m3. The impractical, indeterminable requirement of testing in large enclosures of more than 150 m3 has been removed.

The EN 17272 standard recognizes that a test in an empty enclosure is not equivalent to a test in a room of identical volume containing a load (workstation, equipment, etc.). The standard clearly indicates that its objective is to provide a frame of reference for tests allowing confirmation of efficacy of an automated airborne disinfection system. The standard identifies the fact that each decontamination cycle is unique and that cycles must be validated in practice using appropriate biological indicators or calibrated chemical indicators. This requirement supports the position of the European Chemicals Agency (ECHA) detailed in section 8 of the technical agreement on the efficacy of biocides.
4. Total contact time of airborne disinfection
An automated airborne disinfection cycle is composed of 4 phases:
Conditioning – Preparation and conditioning of the automated airborne disinfection system and of the enclosure to be decontaminated for the purpose of introducing gas, steam or nebulization.
Diffusion –Introduction of gas, steam, or nebulization Contact – The disinfectant agent remains in suspension on the surfaces of the enclosure.
Aeration – Removal of the disinfectant in order to reuse the enclosure.
The NFT 72-281 standard took account solely of the contact time and not the total time during which the disinfectant was in contact with the surfaces of the enclosure. For example, system A could inject 20 g/m3 of disinfectant over a period of one hour and have a contact time of 10 minutes, and system B could inject 20 g/m3 over a period of 15 minutes and have a contact time of 10 minutes. The cycles and the real contact time associated with the surface are very different, but the contact times reported are identical, at 10 minutes. EN 17272 introduces the concept of total contact time of airborne disinfection to represent the total contact time of the disinfectant agent more precisely. This period begins with diffusion of the disinfectant in the enclosure and finishes at the start of aeration.
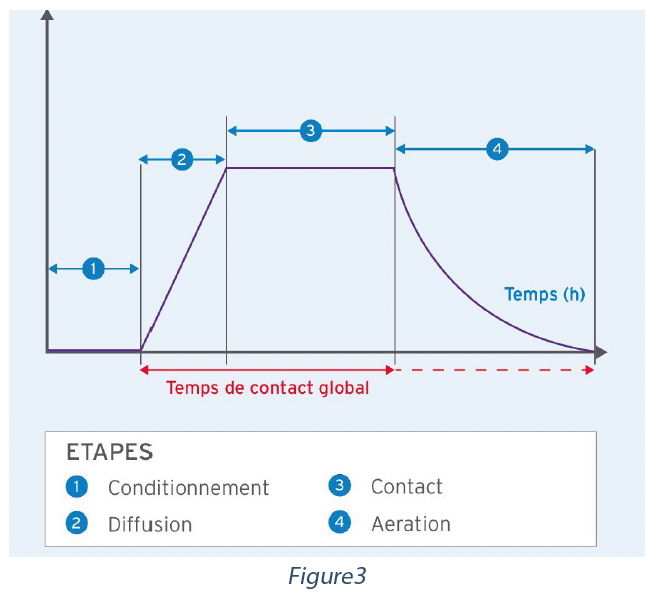
If no airborne process is used, this period finishes at the recovery of biological indicators. If aeration is used, the details of the aeration rates must be included in the test report.
5. EN 17272 Reports
The EN 17272 standard provides instructions on the information to be included in the test report and comprises:
– Identity of the test laboratory
– Name of the product (disinfectant) and the test device
– Type of distribution (vaporization, fogging, nebulization, atomization, etc.)
– Batch number of the disinfectant product
– Serial number of the device
– Type of biological indicator used
– Distance between the device and the indicators
– Scale diagram of the test configuration
– Total contact time
– Diffusion time
– Contact time
– Aeration time
– Aeration rate (or air renewal rate)
– Quantity of disinfectant used
– Exact volume of test enclosure.
The test report must indicate the group(s) of microorganisms which were the subject of tests and indicate whether the test results are “compliant” or “non-compliant” with the requirements of the test. For the distribution test, a reduction of 5-log must be obtained for each biological indicator.
The regulatory bodies and purchasers of automated airborne disinfection systems should request copies of the manufacturer’s EN 17272 test report and examine its test data to determine the conditions under which the system was tested. Manufacturers can claim compliance with the standard EN 17272 on the basis of tests against vegetative bacteria and yeasts. Manufacturers who are looking for a system to treat spores (like Clostridioides difficile), fungi or viruses (like SARS- CoV-2) should check that the system was tested in relation to appropriate test microorganisms to support these claims. Users should evaluate the manufacturer’s claims relative to total contact time, as manufacturers can only declare the product diffusion time (sometimes called application time) or contact time, which may cause underestimation of the total duration of the system cycle.
Conclusion
EN 17272 structures and improves the fundamentals developed by NFT 72-281. The guidance document on efficacy PT 1-5 of the European Biocidal Products Regulation (BPR) identifies the use of the European standard in the evaluation of the applications of biocidal products involving automated airborne disinfection systems.
The Bioquell hydrogen peroxide bio-decontamination system was tested in small and large enclosures according to the EN 17272 standard, for the medical, veterinary, foodstuffs, and industrial fields and supports claims relative to bactericidal, mycobactericidal, sporicidal, fungicidal, yeasticidal and virucidal activity. A range of calibrated biological and chemical indicators is offered, which may be used to check the performance of the airborne disinfection cycle as required in the EN 17272 standard and in the ECHA Technical Agreement for Biocides Efficacy.
Share

John CHEWINS – ECOLAB
john.chewins@ecolab.com


