Summary
- Why and how to carry out thermal characterization of a product before lyophilization?
- How should a risk analysis be conducted for contamination in a lyophilization process?
- CIP evolution in freeze dryers. Optimize turnaround time & water consumption
- Silicon oil detection in pharmaceutical freeze dryer by mass spectrometry
- Mass spectrometers. Experience feedback
- En 17272. Everything you should know about the new standard on airborne room disinfection by automated process
Mass spectrometers.
Experience feedback
Drug manufacturing processes are often complex, not only scientifically, but also when we look at the equipment used. In our industry, we produce delayed release forms using polymer matrices – active principles. During this manufacture, crushing, extrusion and lyophilization processes are used. Each equipment item enables the transformation of raw materials into a pharmaceutical product, but is also a source of contamination.
1. Needs and criteria for the purchase of a spectrometer
In this type of mechanical process, control of equipment wear is a vital factor in limiting contamination. There are equipment parts whose wear can be monitored and which can be changed regularly without too many problems, but when this involves lyophilizers, the situation becomes complicated.
This is costly equipment, used for a batch process, and is often the equipment that limits production capacity. It is expensive and its start-up requires significant time and resources. It has been used for several decades and it is therefore imperative to be able to monitor their wear to ensure product quality.
Lyophilizers are subject to thermal and mechanical constraints. The possibility of loss of integrity of the oil system is one of the main concerns of every manufacturer.
In fact, lyophilizer manufacturers recommend that these hoses be changed, but it is difficult to determine their service life. This depends on too many factors, such as frequency of use, sterilization and cleaning processes, events that occur routinely such as breakdown or accidents. On the advice of the manufacturer, we chose to install a GEA LYOPLUS TM mass spectrometer. The manufacturer offers two variants; an installation fixed on the equipment and another mobile version.
We opted for the choice of a mobile spectrometer, since we had 2 lyophilizers in order to rationalize the investment and ensure back-up in case of breakdown, since it is fairly easy to rent replacement equipment if needed. If the choice of a mobile spectrometer limits purchasing costs, on the other hand great vigilance is required, as this choice is associated with a significant risk of poor measurements and increased risk of damaging the equipment.
2. Measurements on “mobile” LYOPLUS“
To describe the various difficulties in using a mobile spectrometer, we will illustrate the measurement procedure put in place with the precautions for use that are imperative for carrying out reliable measurements without damaging the equipment. The apparatus is on a trolley so allowing it to be moved from one technical area to another to carry out measurements on the different lyophilizers on the site.
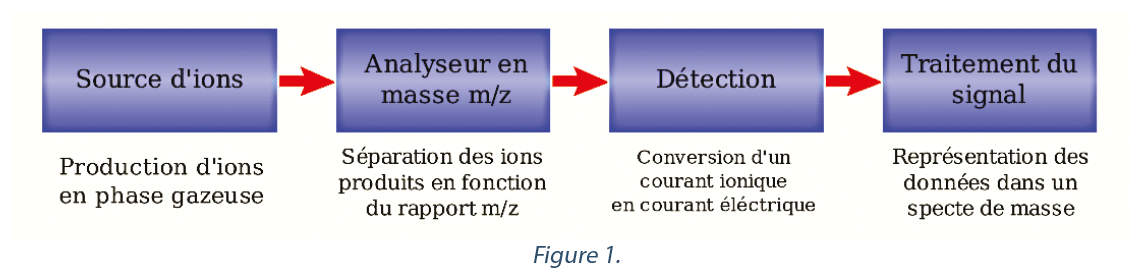
Firstly the functioning of a mass spectrometer should be recalled. Mass spectrometry is a technique that detects and identifies molecules by determining their molecular mass. Its principle lies in the separation in the gas phase of charged molecules according to their mass/charge ratio (m/z). The sample to be identified in gaseous form, in our case the gas (air) present in the lyophilizer, is ionized by a source of ions, these are accelerated, then pass into an analyzer, which sorts the ions according to their m/z ratio, then they reach an ion detector and a computer processes the signal obtained (see Figure1).
Traces of silicone will be detected easily. A simple fingerprint contaminated by silicone will be evaporated and identified by the spectrometer.
To carry out the measurements, the chamber must be at a pressure below 500 microbar. The lower this value, the more the silicone oil vapor will be evaporated and detected, but an overly low value impacts the accuracy of the measurement. The supplier’s recommendations are to carry out the measurement at around 100 microbar. It is also imperative to always carry out measurements under the same conditions in order to be able to compare these with each other.
In our case, this implies ensuring that the lyophilizer is dry when launching the defrosting beforehand. The measurement conditions are the following: 25°C and at 100 microbars, with the valve open between the chamber and the trap . The first 10 minutes are necessary to stabilize the apparatus and are not considered reliable values. Actual measurements are made over 30 minutes and the maximum value measured should be located below the defined action limit. After each measurement, the spectrometer must undergo a sort of cleaning, called “bake”, which involves the heating of the measurement chamber to “burn” the residues of the previous measurement. Subsequently a check of correct operation is conducted by means of a “unit test”, which transmits 4 gas standards with known spectra.
In summary here therefore are the steps:
- Defrosting and drying the lyophilizer
- Placement of the lyophilizer in measurement condition: 100 ± 20 μbar and at 25 ± 2 °C with opening of the valve between the chamber and the trap
- Connection of the lyophilizer to the spectrometer by opening the valve linking both equipment items => careful, the connection must only be made when the conditions of 100 μbar are reached, if not the spectrometer detector will be damaged
- Stabilization for 10 min
- Measurement over 30 min
- Cleaning of the spectrometer (“Bake”)
- Unit test to check the equipment is operating correctly.
As far as possible, the previous steps must be programmed to ensure greater reproducibility and more reliable measurement. The placement of the lyophilizer in operational condition will be distinctly more stable if temperature and pressure are regulated by a robot, than if these parameters are maintained manually. Variability in pressure measurements will affect the value in ppm. Fuller explanations on the subject, as well as on the importance of connections between the mass spectrometer and the lyophilizer will be given in the following chapters.
3. Definitions of specifications
The primary objective of a mass spectrometer coupled to a lyophilizer, is to detect a micro-leak of silicone rapidly in order to prevent any contamination of our products.
To determine a specification, it is important to know the following information:
- The size of the lyophilizer chamber
- The “baseline value” of silicone measured for the lyophilizer in question
- The quantities detected in ppm for a drop of silicone oil (10 mg) or the quantity detected for a micro-leak
- The age of the lyophilizer
Our supplier GEA has carried out a test to determine the number of ppm detected for a drop of silicone oil (10 mg) at 100 μbar added to a lyophilizer on their premises. The test showed that the drop of oil in the 1.3 m3 chamber of the lyophilizer increased the signal by at least 50 ppm. One of our lyophilizers has the same volume and we took this value to calculate our action limit (see below).
On the basis of these elements here are the specifications, which are applied for both lyophilizers on the site.
 |
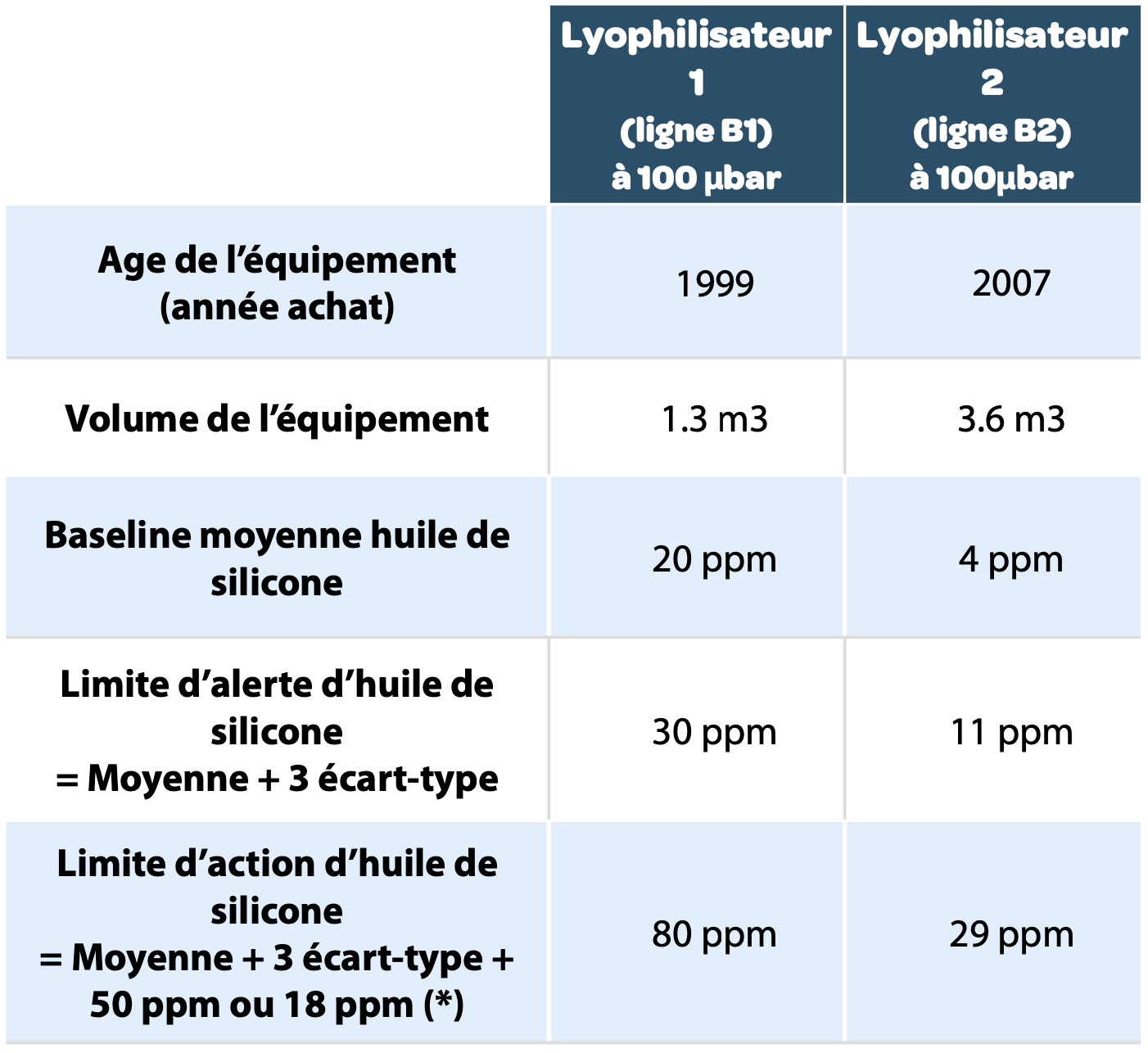 |
* If we consider that the ratio between the size of lyophilizer 2 (3.6m3) and the size of lyophilizer 1(1.3 m3) is 2.8, a drop of silicone oil M5 (10 mg) increases the signal by at least 18 ppm. |
Note that the age of the equipment affects the “baseline”. Older equipment necessarily has a greater level of wear and higher contamination due to regular maintenance.
In addition here are the reference values for traces of silicone oil in a lyophilizer (1.3 m3), given by GEA.
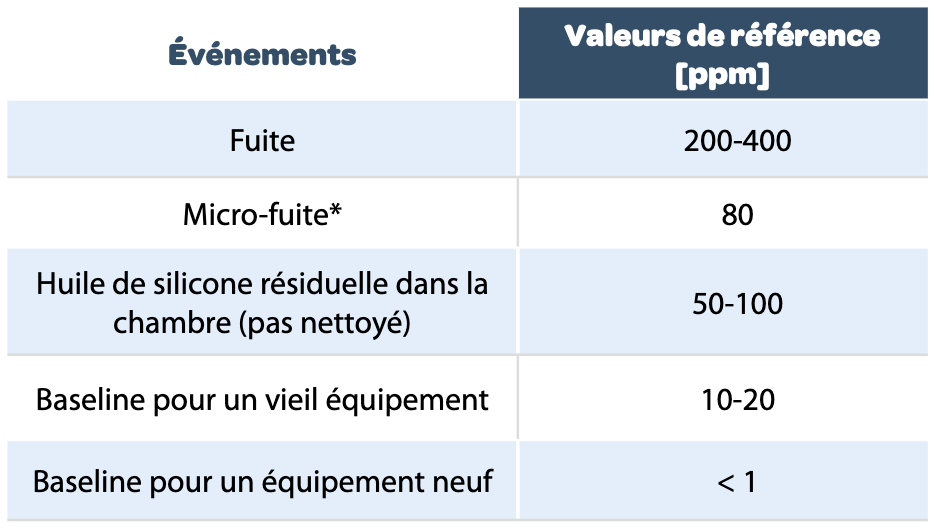 |
4. Results, monitoring
Mass spectrometers also allow a check to be made that the lyophilizer chamber has not been contaminated following maintenance. Contamination by the grease used to lubricate the endless screws in one of our lyophilizers was detected by the mass spectrometer. This allowed us to react before reusing the lyophilizer for production.
5. Cleaning procedure for the lyophilizer chamber after contamination
When the lyophilizer chamber is contaminated with liquid silicone, a cleaning process must be performed. This may be done in different ways according to the level of contamination.
- An appropriate detergent may be used by spraying then rinsing with water
- The performance of several sterilization cycles may also reduce the silicone content in the chamber
- A flush of nitrogen gas in the chamber can also prove effective
Only the measurement of silicone content obtained by the mass spectrometer will provide assurance that the cleaning has been effective.
6. Definition of the frequency of analyses, quality risks / business risks
It would be logical to run an analysis at the end of each batch to check that no traces of silicone oil are present in the lyophilizer, but in practice this is not always possible. The lyophilizer is often a production bottleneck, it is therefore difficult to lose a production slot for a test.
In fact, for the placement of a lyophilizer in operational condition, stabilization, analysis and placement in condition again for the next batch, around 19 hours are needed with much manipulation requiring the presence of a qualified operator.
We have therefore opted for a periodic analysis, at a frequency of 8 batches or at a maximum of 4 weeks.
This time frame was fixed taking into account the lead time of production/ release analyses. The important thing is to be able to detect any contamination before the batch leaves the factory in order to avoid a recall of batches which would have been on the market.
The current quantity and its financial value must be taken into account to take the right decision. Additionally these assessments must be repeated regularly, as the reality of production tends to change particularly with the use of the lyophilizer for new products or changes in production scale. These considerations led us to review our approach and finally to automate measurement to incorporate it in the lyophilizer cycle steps. This avoids having an operator present and carrying out analyses during down time, during the night, etc.
7. Criteria which motivated us to transition to dedicated equipment / fixed on the lyophilizer
Following elevated values obtained on our 2007 (70-180ppm) and 1999 (200-280ppm) lyophilizers, the investigation showed us that the contaminating elements were associated with the equipment used for connecting the spectrometer and particularly the quality of the hose and seals, which contained traces of silicone (even if their presence is not declared in the materials certificate) but also the disassembly and assembly of this connection. The hose was replaced by a braided stainless steel hose and the seals by EPDM seals, but high values were sometimes measured all the same. The action which followed was to have the technician put on gloves before assembling connections, to avoid contamination by finger marks, by hand cream for example, or other elements touched previously. All these measures were beneficial and fresh contamination did not appear. The technician however had to be fully informed of the problem to understand the importance of all these measures and their application.
8.Project to automate analysis sequences in lyophilizer acceptance testing
To minimize all risk, it was decided at Debiopharm that a check of silicone content in the chamber be made with the mass spectrometer before each reuse of the lyophilizer. Measurements of silicone content in the lyophilizer chamber can only be made when the lyophilizer is under clearly defined conditions (trap thawed out, shelf temperature and chamber pressure controlled, etc.).
A number of steps must therefore be executed as soon as the vials have been unloaded from the lyophilizer and before the next load, namely in the order:
- In situ integrity test of the chamber input filter
- Thawing out the trap
- Placement of the lyophilizer in operational conditions (shelf temperature and pressure) for the measurement of silicone content
- Measurement of silicone content by the “Lyoplus” mass spectrometer
- Sterilization cycle
- Cooling of shelves for the next batch (our product being frozen beforehand and introduced into the lyophilizer on precooled shelves). This cooling is programmed for a precise date and time.
The sequence of all the steps is launched automatically following unloading of the batch. As soon as the step is compliant, the next step is launched. If one of the steps is not compliant, the next step is not carried out, an alarm is issued and the intervention group is notified.
The automatic sequence was developed on our PLC / Scada system. This controls the mass spectrometer for measurement of silicone content.
The benefit to the production sector of implementing this automatic sequence is significant. For it is possible to run the different steps in sequence (total duration of 12h) without human intervention and with a minimum stoppage time between unloading the previous batch and the next load (especially if the different steps are done during the night without team working).
Before this automatic sequence was introduced, it was not possible to perform this check of silicone content after the production of each batch.
9. Current situation, monitoring for each batch, trends and alert level.
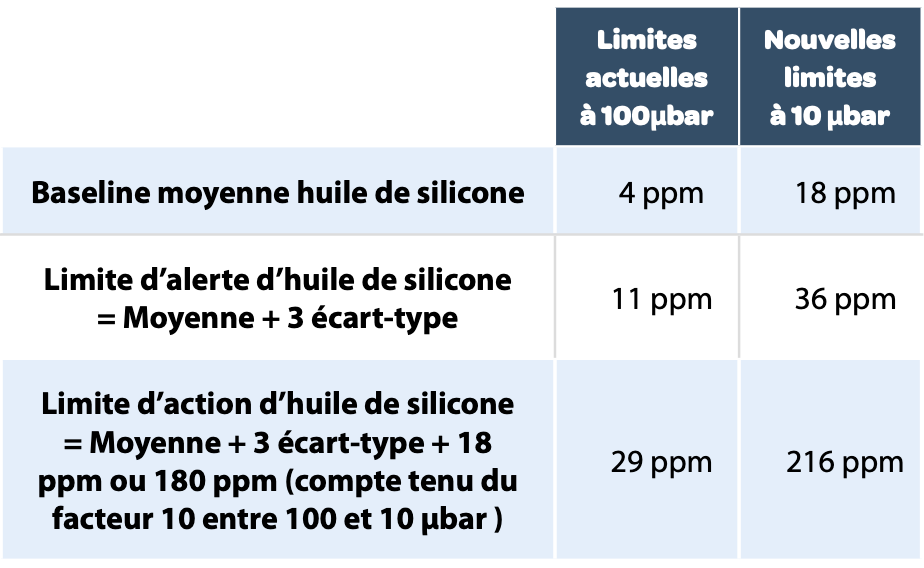
The current measurements are made after each product batch. The values detected are close to 0 ppm at 100 μbar. An investigation is underway to carry out the measurement at 10 μbar and to modify the limits as a consequence.
Here are the new limits proposed for the 2007 lyophilizer 2:
 |
Share

Laurent GAY, Marco SALVAGNI & Nathalie SCHWEIZER – DEBIOPHARM
laurent.gay@debiopharm.com


