Summary
- Outsourcing bioproduction of biomedicines in France
- Outsourcing supplier audits: The keys to success
- Selecting container closure components with confidence: A data-driven approach to CCI
- The Art of Understanding Language: The Evolution of Natural Language Processing
- Microbial Monitoring RABS Gloves: Unravelling the Implications of Directional Use
- General Considerations on Bacterial Endotoxins & USP Approach to Developing GC <86> Bacterial Endotoxins Test Using Recombinant Reagents
- Bacterial Spore Formers in Disinfectant Efficacy Testing
- Avoiding product oxidation by H2O2 in isolators. It all depends on the right analyses!
General Considerations on Bacterial Endotoxins & USP Approach to Developing GC <86> Bacterial Endotoxins Test Using Recombinant Reagents
Bacterial Endotoxins, a component of the Gram-Negative Bacterial (GNB) cell wall, comprise most of the pyrogens found in parenteral manufacturing. Endotoxins are integral with the outer cell membrane of GNB. If GNB cannot grow, endotoxins cannot be generated. However endotoxins may remain active in cell wall fragments after cells die so that a material may be sterile but may still contain quantifiable levels of endotoxins activity.

Bacterial endotoxins, when present in parenteral products (including biological products) or medical devices, indicate that the growth of GNB occurred at some point during the manufacturing steps. Endotoxins can be introduced into the process stream by pharmaceutical ingredients, including water, raw materials (particularly from natural sources), active pharmaceutical ingredients (API), drug product formulation excipients, and primary packaging materials.
The endotoxin testing industry has been exploring new technological advancements to complement traditional methods used in laboratories for endotoxin detection. Patient safety is of the utmost importance, and there is a lot of attention given to finding improvements without introducing new risks, while also ensuring ecological sustainability. In recent years, new techniques have emerged that do not involve animal derivatives or animals, such as the Monocyte Activation test and recombinant reagents, which include recombinant factor C (rFC) and recombinant cascade reagents (rCR). This paper provides an overview of techniques for controlling endotoxin levels and highlights the new USP General Chapter (GC) <86> Bacterial Endotoxins Test Using Recombinant Reagents which introduces the alternative approaches for detecting bacterial endotoxins.
1. Controlling the Level of Endotoxins
The Limulus Amoebocyte Lysate (LAL) test is one of the ways of testing for the presence of endotoxins. This involves incubating a sample to be tested with the lysate of amoebocytes of the horseshoe crab, Limulus polyphemus. This test is simple, rapid, relatively inexpensive, and very sensitive. It can detect pyrogens on the ng/ml level.
For the past 40 years, the LAL test described in the GC <85> Bacterial Endotoxins Test has been the primary method of evaluating parenteral products for the presence of bacterial endotoxins in vitro. The first version of Bacterial Endotoxins Test <85> appeared in USP 20–NF 15 (1980). The chapter was subsequently harmonized with the Japanese and European Pharmacopoeias (JP 4.01, Eur. Ph. 2.6.14), and the first harmonized chapter appeared in USP 25–NF 20 (2002). Since its first publication, the content has changed very little, but years of experience, increasing knowledge, and more complex parenteral formulations suggest that the basic methodologies described could benefit from additional supporting information. Bacterial endotoxin assays may encounter various interferences due to the physical and chemical characteristics of the test sample. If such interferences cannot be overcome or mitigated by accepted levels of sample dilution (i.e., Maximum Valid Dilution) or other validated methods of sample preparation, companies should use the Rabbit Pyrogen Test (RPT) as outlined in GC <151> Pyrogen Tests.
For certain biological products in the U.S., federal regulations still require RPT. The requirement may be waived if a method equivalent to the rabbit pyrogen test is demonstrated following the guidelines in 21 CFR 610.91. It is worth noting that some USP monographs still mandate a rabbit pyrogen test. However, if a company can demonstrate an equivalent pyrogen detection, they may choose to perform an endotoxins test or alternative cell-based test instead. Companies also have the option to use alternative methods and procedures if they provide better accuracy, sensitivity, precision, selectivity, or adaptability to automation or computerized data reduction in other particular circumstances. If such a choice is made, the alternative test for the detection of bacterial endotoxins must be fully validated to ensure that decisions made using the alternative methodology are equivalent to or better than decisions made using the validated USP methods and ultimately approved by the appropriate regulatory authority. Although endotoxin testing is not specifically cited, guidance on validation of alternative methods can be found in the General Chapters Validation of Alternative Microbiological Methods <1223> and Validation of Compendial Procedures <1225>. However, if test results are not conclusive, the referee test is the USP compendial method unless otherwise stated in the monograph for the product being tested. The FDA review division will evaluate the proposed changes on a case-by-case basis.
Concerning the EU, at its 170th session in June 2021, the European Pharmacopoeia (Ph. Eur.) Commission has decided to embark on a path that should lead to the complete replacement of the rabbit pyrogen test (RPT) within approximately five years. The Monocyte-activation test (MAT) was added to the Ph. Eur. in 2009, providing an in vitro alternative to the RPT capable of detecting both endotoxin and non
endotoxin pyrogens. The publication of this chapter was a significant step forward in terms of animal welfare, in accordance with the Council of Europe’s European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes2.
The advantage of the MAT is the capability to detect both endotoxins and non-endotoxin pyrogens. The FDA’s “Guidance for Industry – Pyrogen and Endotoxins Testing: Questions and Answers” mentions this method as an alternative to the Rabbit Pyrogen Test, which must be validated according to USP GC <1225>. Moreover, the USP <151> Pyrogen Test suggests that “A validated, equivalent in vitro pyrogen or bacterial endotoxin test may be used in place of the in vivo rabbit pyrogen test, where appropriate,” and although the MAT is present as a compendial method in Eur. Ph., a complete GMP method validation must be conducted to comply with USP requirements.
2. Proposed General Chapter <86> Bacterial Endotoxin Test Using Recombinant Reagents
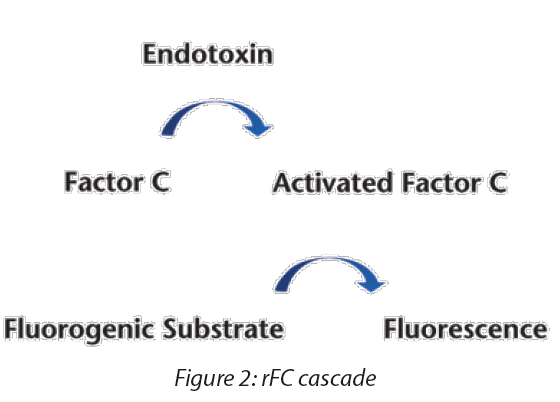
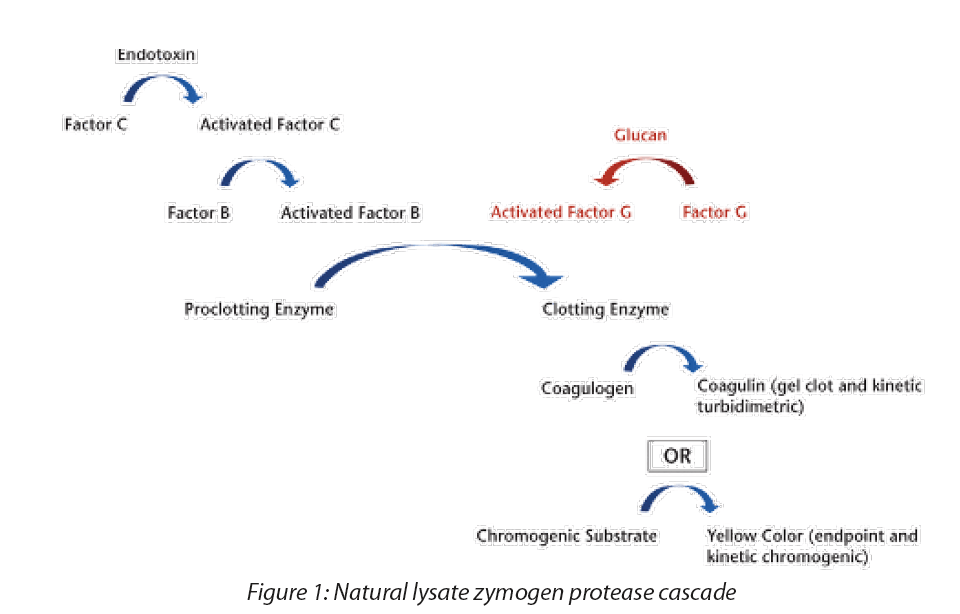
USP published GC <86> Bacterial Endotoxin Test Using Recombinant Reagents in Pharmacopeial Forum 49(6), with a comment period from November 1, 2023 to January 31, 2024. The chapter describes a test method using recombinant reagents, such as recombinant Factor C (rFC) and recombinant Cascade Reagent (rCR), utilizing two detection techniques: the endpoint fluorescence technique and the chromogenic technique. These reagents are an animal-free alternative to the traditional LAL approach. In the horseshoe crab and in the in vitro lysate reaction, endotoxins bind to and convert Factor C to its active form, subsequently activating Factor B, which activates the proclotting enzyme. The activated proclotting enzyme then cleaves the targeted clotting protein, coagulogen, resulting in increased turbidity (kinetic turbidimetric assay) and, ultimately, a clot of the lysate sample mixture (gel clot test). The chromogenic assay is similar to the turbidimetric assay but, rather than cleaving coagulogen, the proclotting enzyme cleaves a chromophore from a colorless substrate, resulting in an increase in color intensity of the reaction mixture. The extent of turbidity or color is proportional to the level of endotoxins activity in the test solution that binds to Factor C, the first zymogen in the cascade (Figure 1).
Recombinant bacterial endotoxins test reagents proposed as alternatives to naturally sourced Limulus amebocyte lysate (LAL) contain one or more recombinant zymogen protease(s) cloned from one or more constituent zymogen elements of the natural cascade. By design, recombinant reagents lack the alternative Factor G pathway where the presence of β-d-glucan can activate Factor G, which in turn can act as a non-endotoxin-specific activator of the proclotting enzyme. When a sample contains sufficient β-d-glucan, the presence of the Factor G pathway in natural lysate can result in an overestimation of endotoxins activity.
Current recombinant Factor C (rFC) reagents contain only the rFC constituent of the clotting cascade. These tests require the use of a qualified and calibrated fluorometer for reading the signal. The rFC test is an endpoint assay and is performed in a manner consistent with GC <85>, Photometric Quantitative Techniques, Chromogenic Technique (Figure 2).
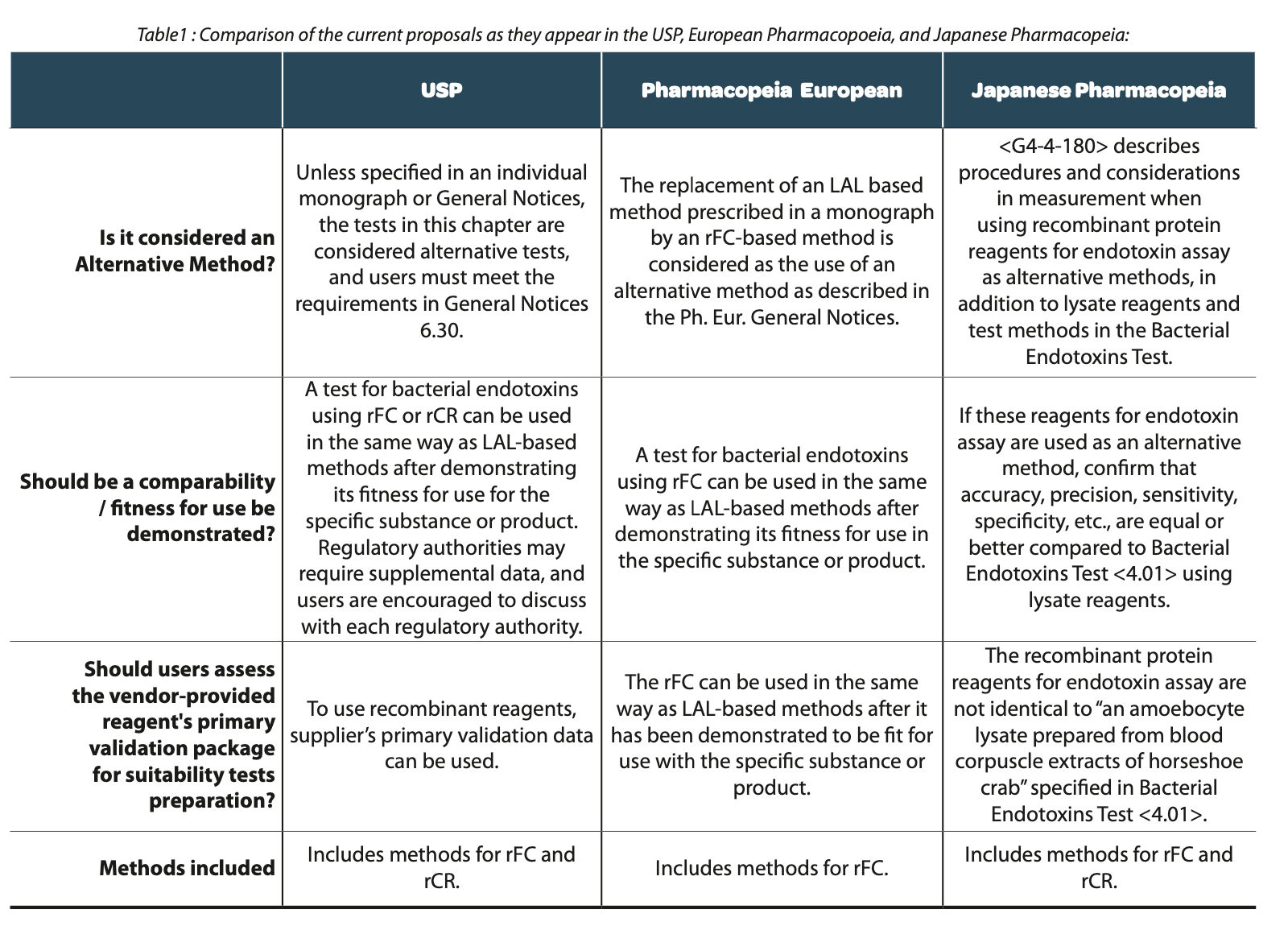
Recombinant cascade reagents (rCR) include all three cloned zymogen proteases (Factor C, Factor B, and the proclotting enzyme) in their formulations as shown in Figure 3. The test methodology for using rCR is the same as the kinetic chromogenic method and is performed as described in GC <85>, Photometric Quantitative Techniques, Chromogenic Technique.
The GC <86> highlights that these tests are considered alternative tests unless specified in an individual monograph and must meet the requirements in General Notices 6.30. This means that users should evaluate the primary validation package for the reagent provided by the vendor and prepare the suitability tests necessary to confirm the method verification for the specific product to which it will be applied. Manufacturers of existing products that choose to use GC <86> need to show comparability to GC <85>, and regulatory authorities may require validation and/or supplemental data. On the other hand, manufacturers of new biopharmaceuticals may be able to evaluate the use GC <86> without comparing to GC <85>. The USP Endotoxin Reference Standard is included and required in this test as in the GC <85>. Users are encouraged to consult each regulatory authority as they may require supplemental data prior to acceptance. An example of supplemental data may include a comparative study of the material tested by techniques described in this chapter and those in GC <85>.
Comparability of recombinant reagents should demonstrate equivalency of results per General Notices 6.30. It is recommended to compare test results using products that contain known levels of measurable endotoxins activity from a source that could reasonably be expected to contaminate the product. The following suggestions are provided to minimize variables that may affect the comparability protocol:
• Given that the recombinant factor C reagent, have no Factor G pathway, the use of a glucan blocker for the lysate reagent is recommended.
• Control standard endotoxins (CSE) are secondary calibration analytes that may be derived from different strains of E. coli and formulated differently among reagent suppliers. The use of one manufacturer’s CSE with another’s reagent may result in a different potency determination that could influence the comparability study outcome (USP GC <1085> Guidelines on Bacterial Endotoxins Test). It is suggested that comparability studies employ the USP Endotoxin Reference Standard for calibration curves and Positive Product Control (PPC) to eliminate any effects that an unmatched combination of reagent lot/CSE lot may have on the test result.
An example of acceptance criteria of measured endotoxins activity using recombinant and naturally derived lysates might be the following: The measured activity of a sample containing endotoxins using a recombinant reagent method should fall within 50%-200% of the measured activity in the same sample tested using natural lysate as described in GC <85>. A sample calculation is provided below for a single sample where endotoxins from autochthonous sources are measured at 4.7 EU/mL using the compendial lysate method and 5.3 EU/mL using the recombinant reagent.
Relative Recovery = [(5.3 EU/mL) ÷ (4.7 EU/mL)] x 100 = 113% recovery
The proposed method in USP-NF, GC <86> Bacterial Endotoxins Test Using Recombinant Reagents is in line with the approaches adopted by the European and Japanese Pharmacopeia. In Addition, USP is proposing to include the rCR and its associated method (which is not present in the current EP chapter), given that multiple manufacturers have recently made it commercially available. (Table1)
3. Conclusions
Recombinant factors are synthetic reagents that provide a sustainable alternative to LAL, which relies on lysate from horseshoe crab species that are endangered in some parts of the world. By using recombinant reagents (rFC and rCR), we can reduce our dependency on these animal resources. Technology providers, subject matter experts, and pharmaceutical companies have conducted comparability studies for several years to determine if the recombinant reagents for BET are as good as, or better than, LAL reagents. With a growing body of evidence and an increase in adopting sustainable alternatives within the life science industry, end-users are increasingly confident in using recombinant reagents as an alternative to LAL reagents to convert to sustainably produced reagents. Changes may be on the horizon, as all major producers of LAL now have their own recombinant versions, likely reflecting the interests of the markets and regulators to shift towards animal-free testing methods. Additionally, the Atlantic fisheries regulators are currently reviewing new harvest limits for horseshoe crabs. It may be necessary to find a sustainable solution to ensure the safety of the horseshoe crab ecosystem as soon as possible. One effective approach could be for companies to use mixed strategies initially, by utilizing recombinant products for in-process testing during their manufacturing cycle. This way, Limulus Amebocyte Lysate (LAL) can be reserved solely for final release testing. Making policy decisions on complex scientific issues to adopt new technologies across diverse agencies is a challenging task, but it is finally underway.
Share
References
1 Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers | FDA. https:// www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry- pyrogen-and-endotoxins-testing-questions-and-answers).
2 European Pharmacopoeia to put an end to the rabbit pyrogen test | ALTEX – Alternatives to animal experimentation. https://altex.org/index.php/altex/announcement/view/321 <85> BACTERIAL ENDOTOXINS TEST DOI: https://doi.org/10.31003/USPNF_M98830_02_01 <1085> GUIDELINES ON ENDOTOXINS TEST
3 DOI: https://doi.org/10.31003/USPNF_M2245_03_01
4 Compendial Notices <86> Bacterial Endotoxins Test Using Recombinant Reagents https://www.uspnf.com/notices/86-bet-using-recombinant-tests-gen-annc-20230822
5 European Directorate for the Quality of Medicines & HealthCare Newsroom Recombinant factorC: new Ph. Eur. chapter available as of 1 July 2020 https://www.edqm.eu/en/-/recombinant-factor-c-new-ph.-eur.-chapter-available-as-of-1-july-2020
6 Bacterial Endotoxins Test and alternative methods using recombinant protein reagents for endotoxin assay 001-1909 https://www.pmda.go.jp/files/000231653.pdf