La Vague 52
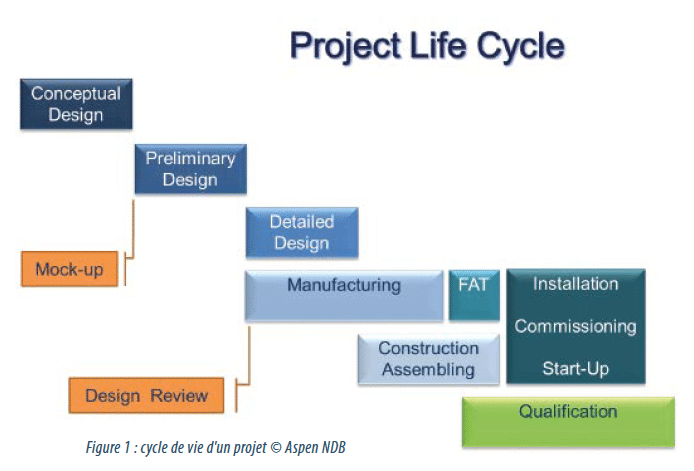
Design of a production isolator. From user need to construction.
It is an absolute truth that all sterile production line installations must be associated with an appropriate means of protection of the product and the environment. When this choice relates to isolator technology, very particular attention must be paid to its design. This must be based on a perfect match between user need and the current state-of-the-art of this cutting-edge technology, culminating in an optimised solution for future production. The installation of a new injectables production line at the Aspen site at Notre-Dame-de-Bondeville (76) is an example. The site, located in Normandy close to Rouen, has specialised in the production of prefilled syringes for many years and produces almost 118 million syringes each year destined for more than 30 international markets including the USA.