Summary
- Quality Assurance & Production: impacts of the new GMP Annex 1.
- Should a visual inspection expert also be a vision system technical expert?
- Residual level of hydrogen peroxide present in isolators during sterility tests: What is the impact on the data generated?
- Advanced Therapy Medicinal Products based on bacteria.
- Supplier & End-User Disinfectant Qualification Comparison for Cleanrooms
- Feedback on the purchase of BFS fillers for a site specializing in PFS.
Residual level of hydrogen peroxide present in isolators during sterility tests: What is the impact on the data generated?
The first isolators made their appearance at the end of the 19th century particularly for the breeding of axenic rodents. In the 20th century, flexible or rigid and chemically sterilizable isolators appeared. In Europe, the company “la Calhène” was a pioneer in this field. Since then, isolators have been used in the worlds of 1) Life sciences 2) Pharmaceuticals 3) Medicine 4) Agri-food 5) Microelectronics.
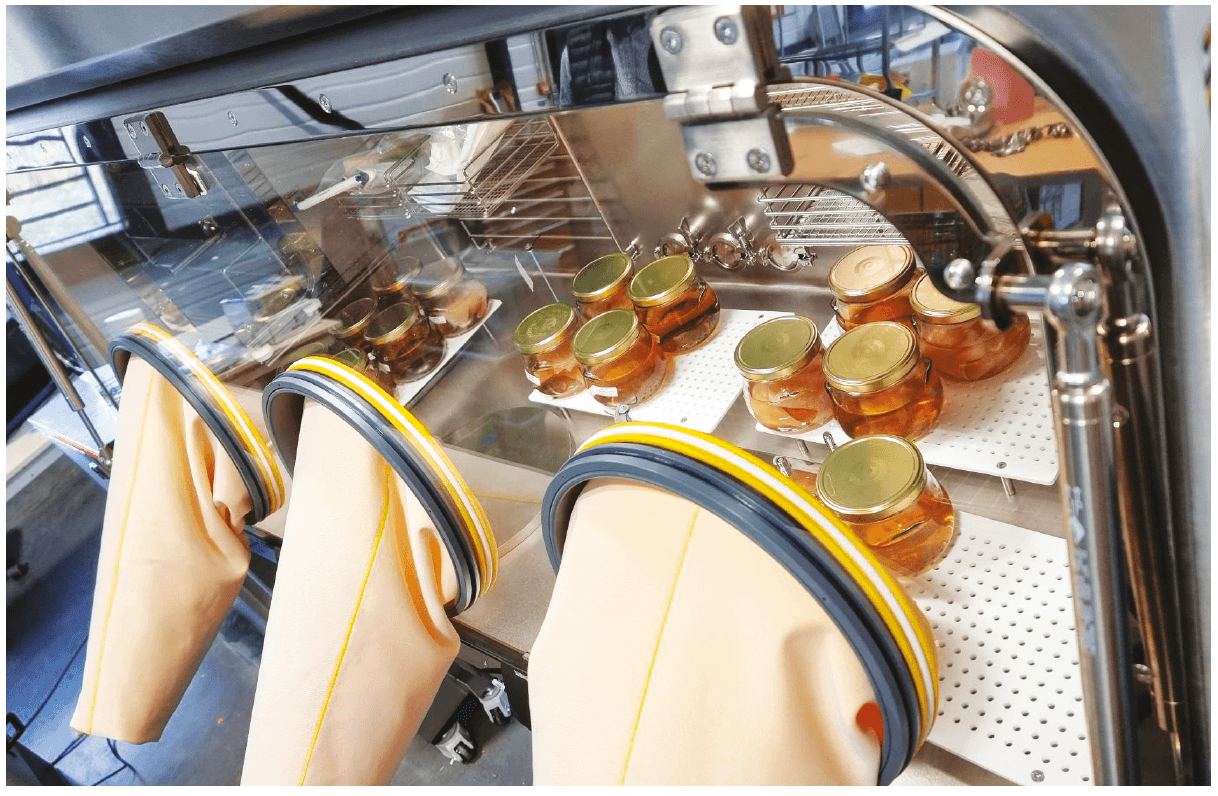
An isolator may be defined as a closed, leak-tight and sterilizable volume, limited by HEPA filters in which operators can intervene while remaining biologically outside this volume. Several functions are allocated to them such as their use as a containment barrier and the treatment and sterilization of air and surfaces. Microbiological contamination of isolators or glove boxes must be eradicated by means of biodecontamination methods via the use of an antimicrobial gas before the start of a process. The biodecontamination process must be validated according to the standard EN ISO 13408-6: 2021.
Two major types of decontamination are currently used, namely peracetic acid and hydrogen peroxide (H2O2). This article will focus on the latter.
1. Hydrogen peroxide as a means of decontamination
Vaporized H2O2 has been used routinely in the pharmaceutical industry since the 1980s. In fact, the bactericidal, virucidal, fungicidal and sporicidal effects of H2O2, as well as its action on bacteriophages are well known and its use in liquid or gaseous form for decontamination was established during the last two decades becoming a standard method in the pharmaceutical industry. H2O2 acts by denaturing proteins through its powerful oxidizing effect, more powerful than that of hypochlorous acid. Nevertheless it presents a certain toxicity to human cells, its cytotoxic mechanism being based on the production of highly reactive OH hydroxyl radicals. Moreover, a pulmonary toxicity threshold at 10 mg/m3 (7.6 ppm) has been demonstrated, with a NOEL (Non Observed Exposure Limit) at 5 mg/m3 (3.8 ppm) in healthy volunteers exposed to aerosols for 5 minutes to 4 hrs (Ranjit, 2020).
In safety data sheet No. 123, the Institut National de la Recherche Scientifique (INRS) reports Occupational Exposure Limit Values authorized over 8 hrs (OELV-8h) in the air of working areas ranging from 0.5 ppm in Germany to 1 ppm (1.5 mg.m-3) in France (INRS, 2022). Thus, it is recommended that over a one-day period workers should not be exposed to more than five times the value of 1 ppm for 15 minutes for a total duration of one and a half hours exposure in the air. However, this exposure value cannot be taken into consideration since the isolator is a closed leak-tight system.
Moreover, this chemical process is a function of the concentration of vaporized H2O2 present in the ambience of the chamber, then condensed at the workload surface. The relative humidity at the start of the cycle, the temperature, the efficacy of mixing and the homogeneity of distribution of the decontaminating fluid are parameters that influence a repeatable and validated biodecontamination cycle. Biodecontamination may be broken down into several phases namely a leak-tightness test, conditioning, injection, stabilization and an aeration phase which reduces the H2O2 concentration to a level compatible with the activities taking place in the isolator. This residual H2O2 concentration potentially affects the active molecules of pharmaceutical products. Consequently, the authorities, as for OELV, recommend weighted exposure limits over time of between 0.5 and 1.0 ppm.
As a reminder, the User Requirement Specifications (URS) for sterility tests for isolators requiring biodecontamination with H2O2 did not previously include any specific requirement for the rinsing phase, not even measurement of the residual level of sterilizing agent. “Appropriate rinsing” was generally stipulated in these URS without mention of the H2O2 concentration in ppm. The important thing was more to check the compatibility of the sterilizing agent with the materials and to show a 6-log spore reduction within the time allocated than to check the residual level. Residual levels of 5, 3 then 1 ppm were then demanded with a rapid rinsing phase without worrying about desorption from polymer or Tyvek packaging. Nevertheless to achieve these low residual concentrations values, the rinsing and waiting time may be long generating immobilization costs.
2. Aim of the study
Based on paragraph 2.6.1. of the European Pharmacopoeia (2020), the aim of this preliminary study was to assess whether a sterility test generated false negatives when it was performed in an isolator with a residual H2O2 level above 1 ppm, following its biodecontamination.
3. Materials and Methods
3.1 Equipment used
a. Isolator used
To do this three sterility tests at a residual H2O2 concentration of 5 ppm were performed in a 3 glove isolator incorporating a biodecontamination airlock. A reference test at a residual H2O2 concentration of 0 ppm was also performed, the residual concentration being measured by a Dräger sensor H2O2 LC.
b. Products to be tested
These sterility tests were performed on sterile wipes (Ecolab KlerwipeTM Product reference 3035500).
c. Preparation and sterilization of the culture media used
The sterility of each batch of culture medium made was ensured in compliance with the standard EN ISO 17665-1: 2006, “Sterilization of health care products”.
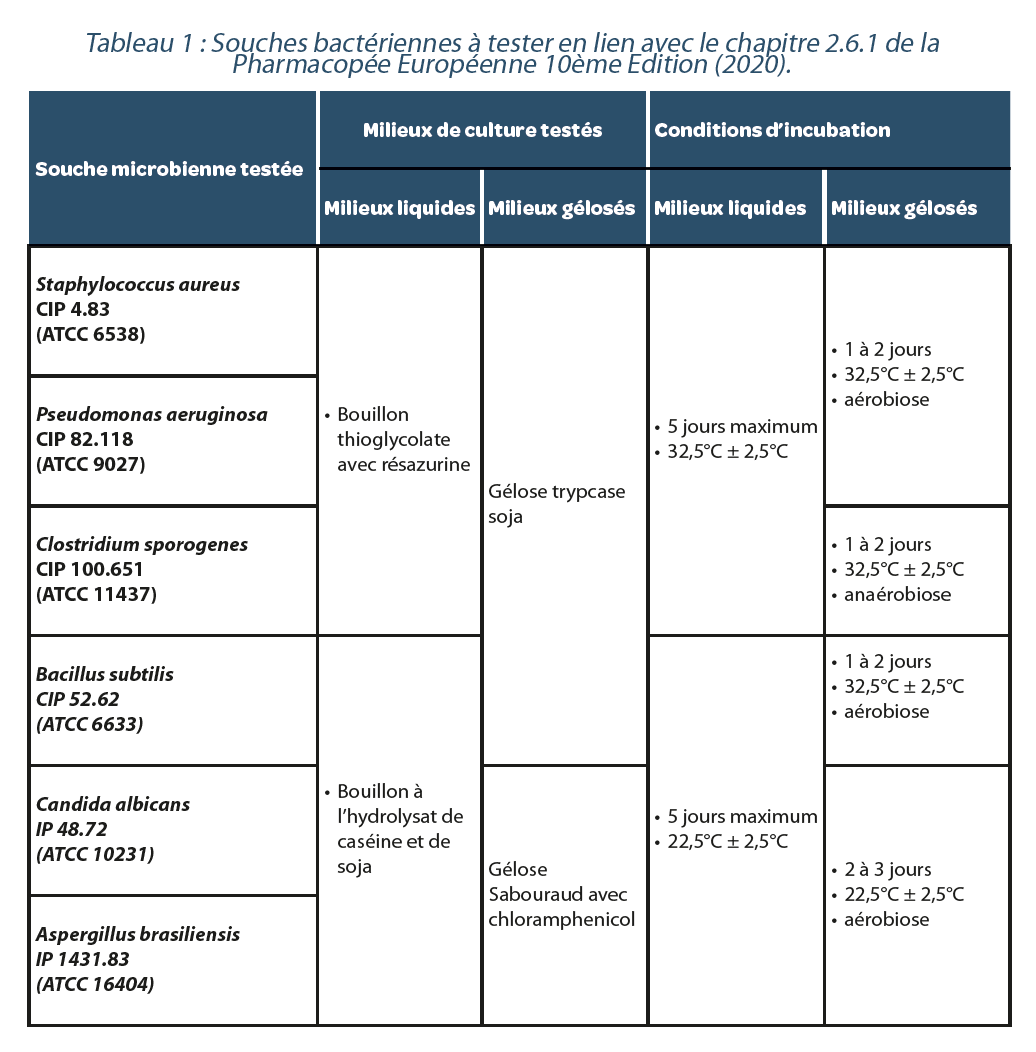
The fertility of each batch of culture medium was checked before use in accordance with the paragraph “effectiveness of culture media” on testing, chap. 2.6.12 of the European Pharmacopoeia 10th Edition (2020), the culture media used being Trypticase-soy agar, Sabouraud agar with chloramphenicol, casein hydrolysate and soy broth, Thioglycollate broth with resazurin.
d. Microorganismes testés et conditions de culture
Table 1 presents the microorganisms tested and their culture conditions. These microorganisms were used in the form of calibrated inocula, titrated at between 10 and 100 CFU /0.1ml-1, developed by the company ICARE (see Table 1).
3.2 Methods
a. Preparation
For each sterility test the following were placed in the isolator before decontamination:
– 8 jars containing 500 ml of casein hydrolysate and soy broth
– 8 jars containing 500 ml of thioglycollate broth with resazurin
– 1 packet of 10 sterile wipes
– 8 pairs of sterile forceps
– 1 20-200 μl micropipette packaged in a sterile sachet
– 1 box of 200 μl micropipette tips packaged in a sterile sachet
Once this equipment was deposited, 3 Geobacillus stearothermophilus ATCC 7953 biological indicators with 106 spores/biological indicator were placed in the isolator. In fact, the European Ph. 10th edition and the USP 31 recommend the use of Geobacillus stearothermophilus for validation of the sterilization method, as it is the strain most resistant to H2O2. It is more resistant than Bacillus subtilis spores, Bacillus being gram+ aerobic bacteria that produce endospores known for their high level of resistance to heat, to chemical products and to dryness. Their viability in low nutrient media is also well known. An H2O2 biodecontamination cycle of the isolator chamber was then started.
b. Biodecontamination of the isolator
The biodecontamination system used for this study was a STELEC* H2O2 generator that vaporizes H2O2 via a thermal action. The decontamination agent 35% H2O2 (RAIDOX) was vaporized via a vector gas in the volume to be biodecontaminated according to predefined parameters incorporating time, pressure, humidity level, temperature, volume and the rate and speed of injection, which allowed a 6-log spore destruction to be achieved. The duration of the biodecontamination phase for this test was 60 min.
A continuous recording of the following parameters was performed via HMI (Siemens, France): humidity level, quantity of sterilizing agent, evaporation temperature, diffusion temperature, diffusion time, contact and aeration time, diffusion pressure, flow of diffused air, high and low H2O2 concentration.
The isolator was ventilated for a duration of 10 minutes via a catalytic system until the target concentration of residual H2O2 to be tested, that is 5%, was reached. Following these operations, a sterility test was performed.
c. Performance of the sterility test
As soon as the target concentration of residual H2O2 was reached in the isolator (5ppm), sterility tests by direct inoculation were performed following the methodology in paragraph 2.6.1. of the European Pharmacopoeia (2020): for each microorganism tested, a jar of culture medium appropriate for the microorganism tested was opened for 6 seconds in order to place a sterile wipe inside (= Tests), another jar was opened then closed again without placing a sterile wipe inside (=Validation controls). It should be reported that this contact time with the air is identical to what may be recorded during sterility tests by direct inoculation performed in laboratories.
For each type of culture medium used, negative controls were run in the same way as Tests and Validation controls (= Test negative controls and Validation negative controls respectively). All jars of culture media were then closed again pending complete ventilation of the isolator.
During the return to a residual H2O2 concentration of 0 ppm in the isolator (around 15 minutes), calibrated inocula of the microbial strains to be tested were placed in the isolator transfer airlock, packaged in a sterile plastic bag. A decontamination cycle of the H2O2 transfer airlock was then performed before passing the calibrated inocula into the isolator chamber. This decontamination cycle lasted 75 minutes.
Ten to 100 microorganisms of each microbial strain to be tested (see Table 1) were then inoculated, each into one of the jars of appropriate culture medium containing a wipe (“Test”) and into an appropriate culture medium receptacle without a wipe (“Validation control”). The “Test negative controls” and “Validation negative controls” were not contaminated by calibrated inocula.
d. Tests performed
Simultaneously, for each microbial strain tested, a test of the number of microorganisms inoculated was performed by counting on an agar plate appropriate for each microorganism (see Table 1).
This was performed by depositing the volume used in μl of calibrated inocula of each microorganism on the appropriate agar culture medium (2 dishes per strain).
For each decontamination cycle performed, Geobacillus stearothermophilus ATCC 7953 biological indicators were placed in the isolator to check the efficacy of the disinfection process, as well as an unexposed biological indicator (positive control). These biological indicators were cultured in tubes of liquid casein hydrolysate and soy medium (Spordex culture media, STERIS).
e. Incubation
After performance of the sterility tests by direct inoculation, the jars of liquid media and agar culture media were incubated in ovens at controlled temperatures, in accordance with the culture conditions set out in Table 1. For the bacterial strain Clostridium sporogenes ATCC 11437, the agar culture media were placed in hermetically sealed plastic sachets, containing anaerobic generators (GENbag anaer kit, Biomérieux) and anaerobic indicators (ThermoScientific).
For each decontamination cycle performed, the Geobacillus stearothermophilus ATCC 7953 biological indicators placed in the isolator to monitor the efficacy of the disinfection process, and an unexposed biological indicator (positive control), were cultured in tubes of liquid casein hydrolysate and soy medium (Spordex culture media, STERIS) and incubated at 58°C ± 2°C for 7 days.
4. Results
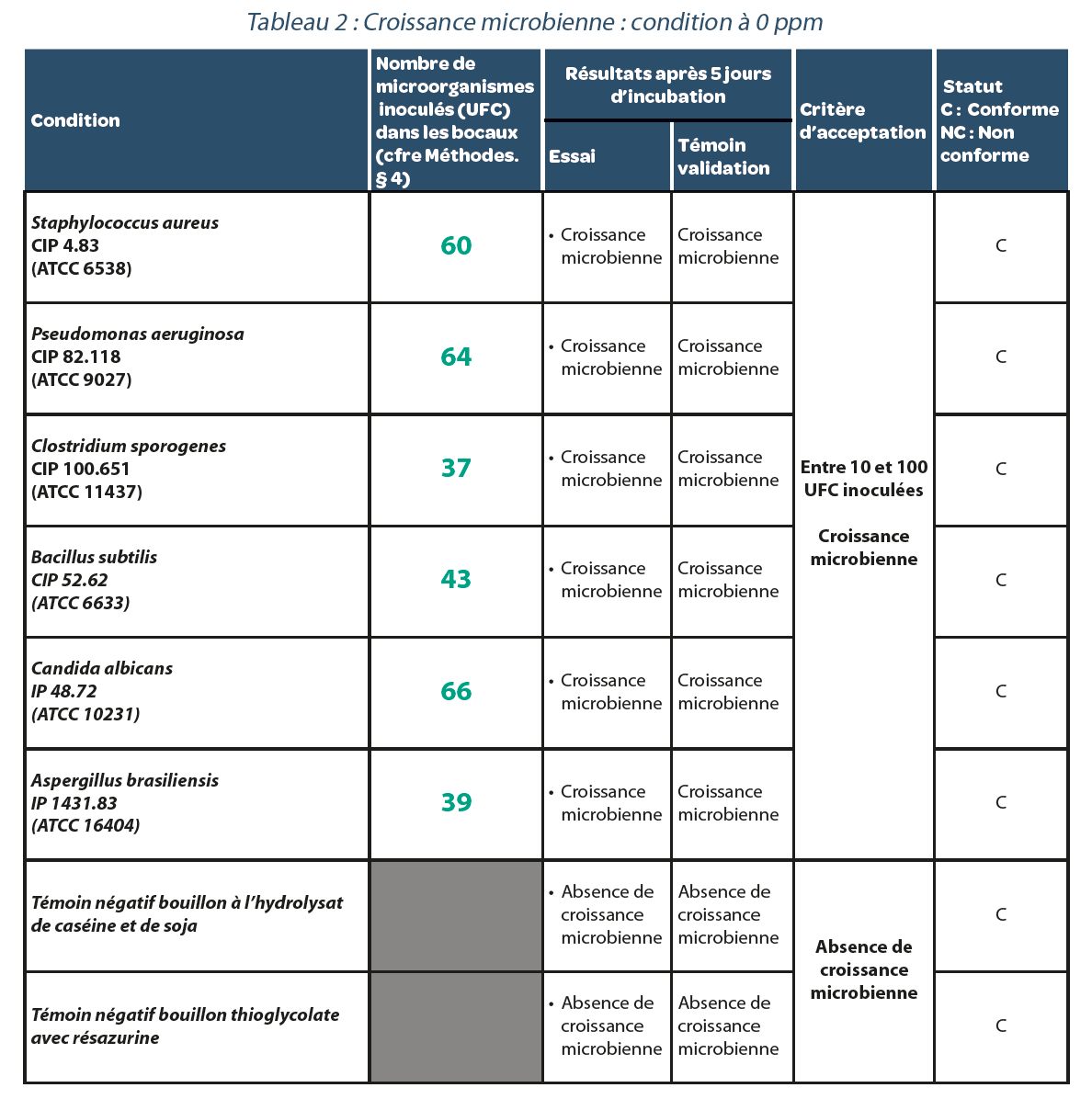
4.1. Reference test. Validation of a sterility test method in an isolator decontaminated with H2O2.
Residual value after decontamination: 0 ppm.
After 5 days of incubation, (Table 2), each bacterial strain studied gave rise to microbial growth. Thus a sterilization cycle in a decontaminated isolator with a residual H2O2 concentration of 0 ppm does not generate false negatives.
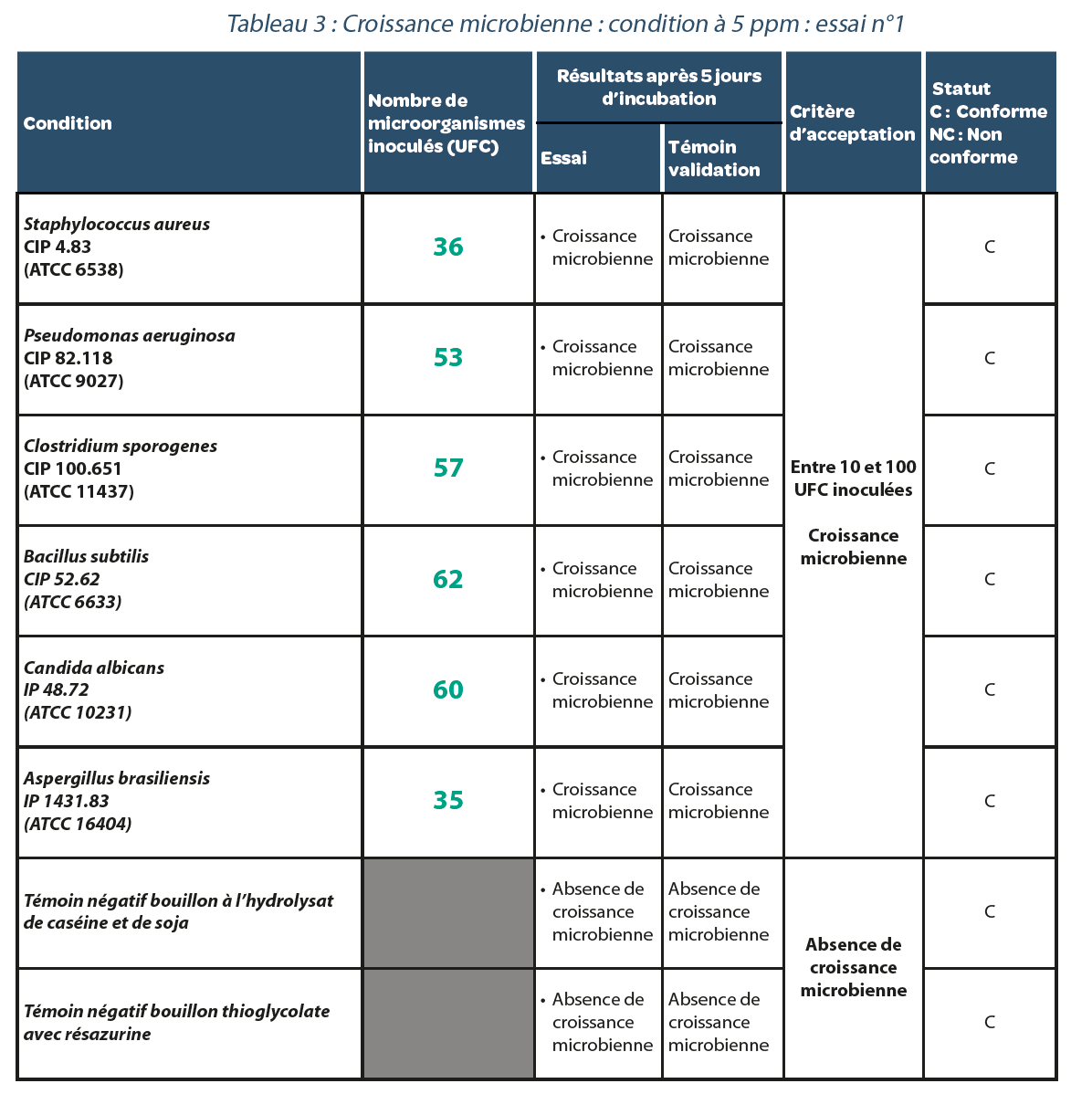
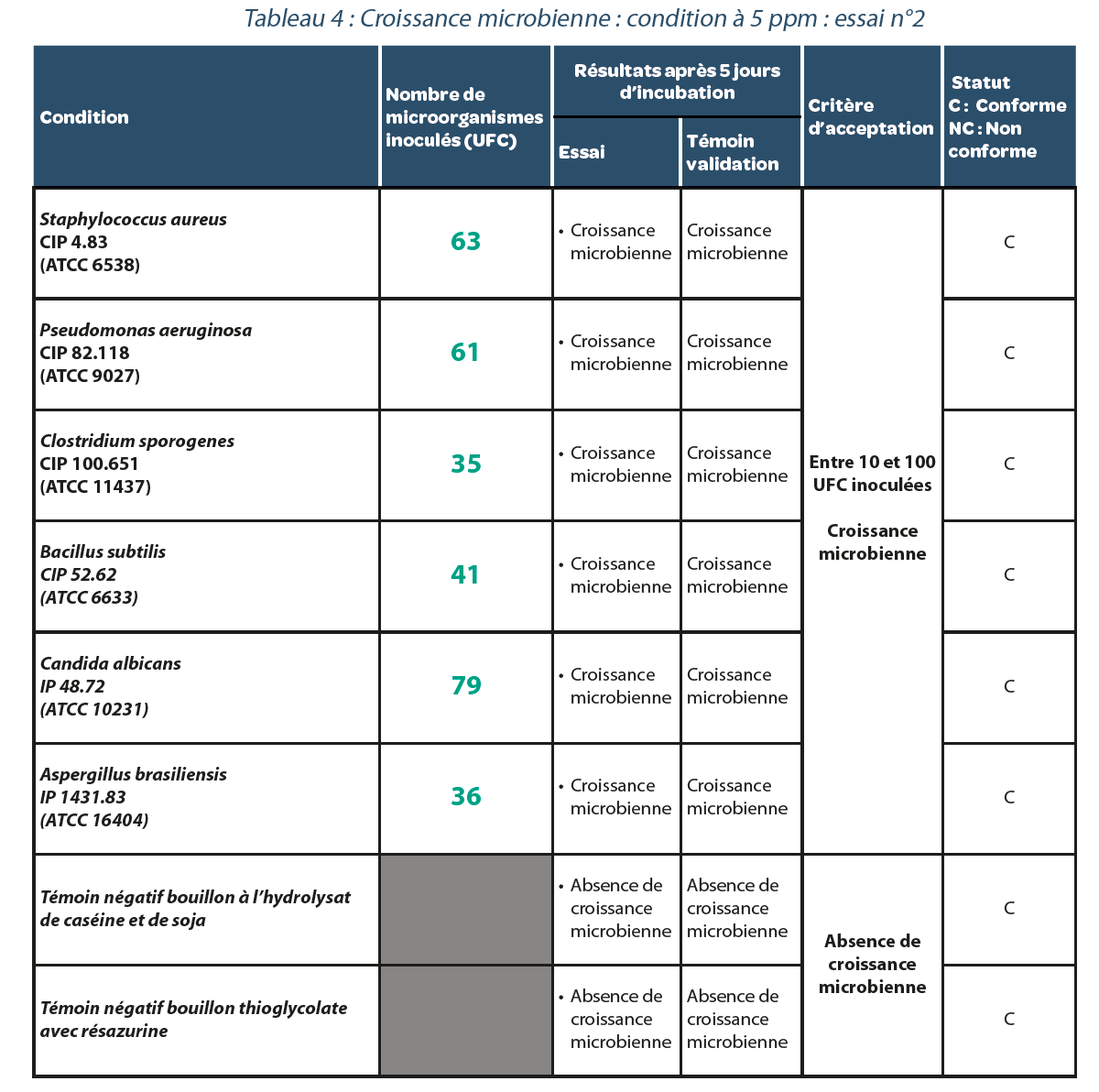
4.2. Validation of a sterility test method in an isolator decontaminated with H2O2.
Residual value after decontamination: 5 ppm.
Three replicates were performed. The results are carried forward in Tables, 3, 4 and 5. As in the previous case, for each repeat of the experiment with a residual H2O2 concentration of 5 ppm in the isolator, all bacterial strains studied presented microbiological growth suggesting that no false negatives were generated under these conditions.
4.3. Biodecontamination
Figure 1 represents a biodecontamination cycle. The rise in concentration at the start of rinsing is due to desorption of H2O2. It should be noted that, for each decontamination cycle performed, the biological indicators did not display growth, which reflects the efficacy of biodecontamination.
5. Discussion & conclusion
In numerous pharmaceutical applications, control of contamination risks is a crucial issue. These activities require areas classified as clean zones according to the standard ISO 14644. The
pharmaceutical industry therefore uses controlled atmosphere areas (CAA) requiring control of microbial contamination risks and biodecontamination processes. Isolators represent one of these CAA. Decontamination processes are based on high concentrations of hydrogen peroxide (up to 35%) in a closed environment. Moreover, as all decontamination cycles must be validated, the choice of biological indicators is strategic in order to obtain “worst case” data. The bacterial strain most commonly used with H2O2 is the strain Geobacillus stearothermophilus ATCC 12980, a strain that we used during the biodecontamination process.
Our study shows that under the experimental conditions, the sterility tests performed in an isolator with a residual H2O2 value of 5 ppm do not seem to impact sterility tests and therefore generate false negatives. This result is very valuable to the extent that the rinsing time needed to return to H2O2 concentrations < 1 ppm (discharge, desorption from packaging…) may generate production shutdowns so increasing costs. We deliberately chose to use sterility tests by direct inoculation, a test that is recommended for solid medical devices.
Nevertheless, it would be appropriate to perform this same study using a filtration method, the most common method in pharma. In the same way, other experiments are necessary to evaluate the limit value of residual H2O2 concentrations.
Share article

Acknowledgements
All of these tests were performed in collaboration with the company ICARE, located in Saint Beauzire (63), which has considerable expertise in the control of the safety of health care products.
References
- References
- Doriath C. Cycle H2O2: acceptation paramétrique pour remplissage aseptique. La Vague. 2009, 28: 7-10.
- Guide de l’Ultra-Propreté 2008-2009, 6th edition, BCMI, Neuilly-sur-Seine, 2008.
- INRS. Peroxyde d’hydrogène et solutions aqueuses. Fiche toxicologique, n° 123. 2022.
- Kleinmann S, Scheu M. Advanced vaporized H2O2 decontamination technology for pharmaceutical isolators: Reduction of H2O2 decontamination cycle time using direct injection nozzles. La Vague. 2017, 52: 31-38.
- Meyer D. Barrières: le confinement souple appliqué aux systèmes de protection rapprochée. Salles Propres. 1979. n°55: 23-26.
- Mounier C. Maîtrise des paramètres et des aléas d’une biodécontamination à l’H2O2. Salles Propres, 2010, 69: 1-5.
- European Pharmacopoeia 10th Edition. Chap. 2.6.1. Editor: Council of Europe; 2020
- Ranjit N. Mesure des résidus en peroxyde d’hydrogène après stérilisation basse température : existe-t-il un risque. Sciences Pharmaceutiques, 2020.
✻